Objectives
- Define renal blood flow, renal plasma flow, glomerular filtration rate, and filtration fraction, and give normal values.
- State the formula relating flow, pressure, and resistance in an organ.
- Identify the successive vessels through which blood flows after leaving the renal artery.
- State the relative resistances of the afferent arterioles and efferent arterioles.
- Describe how changes in afferent and efferent arteriolar resistances affect renal blood flow.
- Describe the 3 layers of the glomerular filtration barrier and define podocyte, foot process, and slit diaphragm.
- Describe how molecular size and electrical charge determine filterability of plasma solutes; state how protein binding of a low-molecular-weight substance influences its filterability.
- State the formula for the determinants of glomerular filtration rate, and state, in qualitative terms, why the net filtration pressure is positive.
- State the reason glomerular filtration rate is so large relative to filtration across other capillaries in the body.
- Describe how arterial pressure, afferent arteriolar resistance, and efferent arteriolar resistance influence glomerular capillary pressure.
- Describe how changes in renal plasma flow influence average glomerular capillary oncotic pressure.
- Define autoregulation of renal blood flow and glomerular filtration rate.
Renal Blood Flow
![]() The amount of blood flowing through the kidneys is huge relative to their size. Renal blood flow (RBF) is about 1 L/min. This constitutes 20% of the resting cardiac output through tissue that constitutes less than 0.5% of the body mass! Considering that the volume of each kidney is less than 150 mL, this means that each kidney is perfused with over 3 times its total volume every minute. All of this blood is delivered to the cortex. About 10% of the cortical blood flow is then directed to the medulla.
The amount of blood flowing through the kidneys is huge relative to their size. Renal blood flow (RBF) is about 1 L/min. This constitutes 20% of the resting cardiac output through tissue that constitutes less than 0.5% of the body mass! Considering that the volume of each kidney is less than 150 mL, this means that each kidney is perfused with over 3 times its total volume every minute. All of this blood is delivered to the cortex. About 10% of the cortical blood flow is then directed to the medulla.
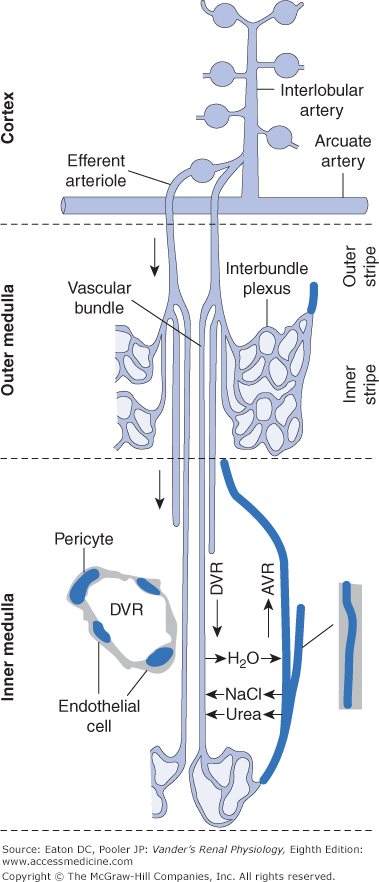
Blood enters each kidney at the hilum via a renal artery. After several divisions into smaller arteries blood reaches arcuate arteries that course across the tops of the pyramids between the medulla and cortex. From these, cortical radial arteries project upward toward the kidney surface and give off a series of afferent arterioles (AAs), each of which leads to a glomerulus within Bowman’s capsule (Figure 2–1). These arteries and glomeruli are found only in the cortex, never in the medulla. In most organs, capillaries recombine to form the beginnings of the venous system, but the glomerular capillaries instead recombine to form another set of arterioles, the efferent arterioles (EAs). The vast majority of the EAs soon subdivide into a second set of capillaries called peritubular capillaries. These capillaries are profusely distributed throughout the cortex intermingled with the tubular segments. The peritubular capillaries then rejoin to form the veins by which blood ultimately leaves the kidney.
Figure 2–1.
The renal microcirculation. Arcuate arteries run just above the corticomedullary border, parallel to the surface, and give rise to cortical radial (interlobular) arteries radiating toward the surface. Afferent arterioles originate from the cortical radial arteries at an angle that varies with cortical location. Blood is supplied to the peritubular capillaries of the cortex from the efferent flow out of superficial glomeruli. Blood is supplied to the medulla from the efferent flow out of juxtamedullary glomeruli. Efferent arterioles of juxtamedullary glomeruli give rise to bundles of descending vasa recta in the outer stripe of the outer medulla. In the inner stripe of the outer medulla, descending vasa recta and ascending vasa recta returning from the inner medulla run side by side in the vascular bundles, allowing exchange of solutes and water as described in Chapter 6. Descending vasa recta from the bundle periphery supply the interbundle capillary plexus of the inner stripe, whereas those in the center supply blood to the capillaries of the inner medulla. Contractile pericytes in the walls of the descending vasa recta regulate flow. AVR, ascending vasa recta; DVR, descending vasa recta. (Used with permission from Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253–F266.)
EAs of glomeruli situated just above the corticomedullary border (juxtamedullary glomeruli) do not branch into peritubular capillaries the way most EAs do. Instead these arterioles descend downward into the outer medulla. Once in the medulla they divide many times to form bundles of parallel vessels called vasa recta (Latin recta for “straight” and vasa for “vessels”). These bundles of vasa recta penetrate deep into the medulla (see Figure 2–1). Vasa recta on the outside of the vascular bundles “peel off” and give rise to interbundle networks of capillaries that surround Henle’s loops and the collecting ducts in the outer medulla. Only the center-most vasa recta supply capillaries in the inner medulla; thus, little blood flows into the papilla. The capillaries from the inner medulla re-form into ascending vasa recta that run in close association with the descending vasa recta within the vascular bundles. The structural and functional properties of the vasa recta are rather complex, and will be elucidated further in Chapter 6.
Blood flow through the vasa recta into the medulla is far less than cortical blood flow, perhaps 0.1 L/min. Although low relative to cortical blood flow, medullary blood flow is not low in an absolute sense and is quite comparable to blood flow in many other tissues. The significance of the differences between cortical and medullary blood flow and the vascular anatomy is the following: The high blood flow and peritubular network in the cortex maintain the interstitial environment of the cortical renal tubules very close in composition to that of blood plasma throughout the body. In contrast, the lower blood flow and grouping of vascular bundles in the medulla permit an interstitial environment that is quite different from blood plasma. As described in Chapter 6, the interstitial environment in the medulla plays a crucial role in regulating water excretion.
Flow, Resistance, and Blood Pressure in the Kidneys
![]() Blood flow in the kidneys obeys the same hemodynamic principles as found in other organs throughout the body. The basic equation for blood flow through any organ is as follows:
Blood flow in the kidneys obeys the same hemodynamic principles as found in other organs throughout the body. The basic equation for blood flow through any organ is as follows:
where Q is organ blood flow, ΔP is the mean pressure in the artery supplying the organ minus mean pressure in the vein draining that organ, and R is the total vascular resistance in that organ. The resistance of an organ is determined by the resistance of individual vessels and their series/parallel connections. The resistance of any single vessel is a function of blood viscosity, vessel length, and most of all, vessel radius. As described by Poiseiulle’s law, the resistance of a cylindrical vessel varies inversely with the fourth power of vessel radius. It takes only a 19% decrease or increase in vessel radius to double or halve vessel resistance. Because the kidneys contain so many parallel pathways, that is, glomeruli and associated vessels, total renal vascular resistance is low. In turn this accounts for the high RBF.
The presence of 2 sets of arterioles (afferent and efferent) and 2 sets of capillaries (glomerular and peritubular) makes the vasculature of the cortex unusual. The resistances of the afferent and EAs are about equal in most circumstances and account for most of the total renal vascular resistance. Resistances in arteries preceding AAs (ie, cortical radial arteries) and in the capillaries play some role, but we concentrate on the arterioles because these resistances are variable and are the sites of regulation. A change in the resistance of an AA or EA has the same effect on blood flow because these vessels are in series. When the 2 resistances both change in the same direction (the most common state of affairs), their effects on RBF are additive. When they change in different directions—one resistance increasing and the other decreasing—the changes offset each other.
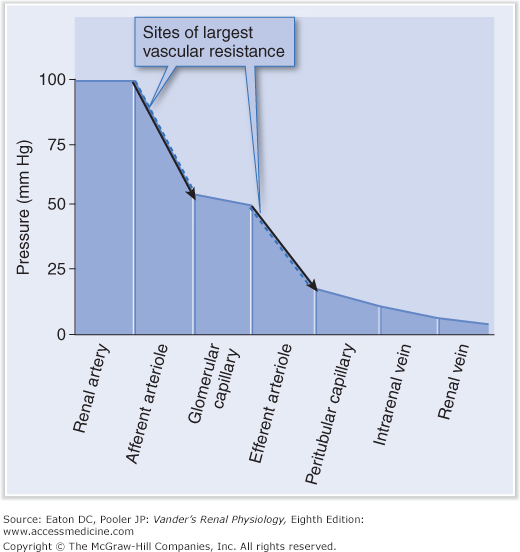
Hydrostatic pressures are much higher in the glomerular capillaries than in the peritubular capillaries. As blood flows through any vascular resistance the pressure progressively decreases. Pressure at the beginning of a given AA is close to mean systemic arterial pressure (~100 mm Hg) and decreases to about 60 mm Hg at the point where it feeds a glomerulus. Because each glomerulus contains so many capillaries in parallel, pressure decreases very little during flow through those capillaries and glomerular capillary pressure stays close to 60 mm Hg. Then pressure decreases again during flow through an EA, to about 20 mm Hg at the point where it feeds peritubular capillaries (Figure 2–2). The high glomerular pressure of about 60 mm Hg is necessary to drive glomerular filtration, whereas the low peritubular capillary pressure of 20 mm Hg is equally necessary to permit the reabsorption of fluid from the renal interstitium.
Figure 2–2.
Blood pressure decreases as blood flows through the renal vascular network. The largest drops occur in the sites of largest resistance—the afferent and efferent arterioles. The location of the glomerular capillaries, between the sites of high resistance, results in their having a much higher pressure than the peritubular capillaries. (Reproduced with permission from Kibble J, Halsey CR. The Big Picture: Medical Physiology. New York: McGraw-Hill; 2009.)
Glomerular Filtration
![]() The glomerular filtrate contains most inorganic ions and low-molecular-weight organic solutes in virtually the same concentrations as in the plasma. It also contains small plasma peptides and a very limited amount of albumin. Filtered fluid must pass through a 3-layered glomerular filtration barrier. The first layer, the endothelial cells of the capillaries, is perforated by many large fenestrae (“windows”), like a slice of Swiss cheese, which occupy about 10% of the endothelial surface area. They are freely permeable to everything in the blood except cells and platelets. The middle layer, the capillary basement membrane, is a gel-like acellular meshwork of glycoproteins and proteoglycans, with a structure like a kitchen sponge. The third layer consists of epithelial cells (podocytes) that surround the capillaries and rest on the capillary basement membrane. The podocytes have an unusual octopus-like structure. Small “fingers,” called pedicels (or foot processes), extend from each arm of the podocyte and are embedded in the basement membrane (see Figure 1–5D). Pedicels from a given podocyte interdigitate with the pedicels from adjacent podocytes. The pedicels are coated by a thick layer of extracellular material, which partially occludes the slits. Extremely thin processes called slit diaphragms bridge the slits between the pedicels. Slit diaphragms are widened versions of the tight junctions and adhering junctions that link all contiguous epithelial cells together and are like miniature ladders. The pedicels form the sides of the ladder, and the slit diaphragms are the rungs. Spaces between slit diaphragms constitute the path through which the filtrate, once it has passed through the endothelial cells and basement membrane, travels to enter Bowman’s space.
The glomerular filtrate contains most inorganic ions and low-molecular-weight organic solutes in virtually the same concentrations as in the plasma. It also contains small plasma peptides and a very limited amount of albumin. Filtered fluid must pass through a 3-layered glomerular filtration barrier. The first layer, the endothelial cells of the capillaries, is perforated by many large fenestrae (“windows”), like a slice of Swiss cheese, which occupy about 10% of the endothelial surface area. They are freely permeable to everything in the blood except cells and platelets. The middle layer, the capillary basement membrane, is a gel-like acellular meshwork of glycoproteins and proteoglycans, with a structure like a kitchen sponge. The third layer consists of epithelial cells (podocytes) that surround the capillaries and rest on the capillary basement membrane. The podocytes have an unusual octopus-like structure. Small “fingers,” called pedicels (or foot processes), extend from each arm of the podocyte and are embedded in the basement membrane (see Figure 1–5D). Pedicels from a given podocyte interdigitate with the pedicels from adjacent podocytes. The pedicels are coated by a thick layer of extracellular material, which partially occludes the slits. Extremely thin processes called slit diaphragms bridge the slits between the pedicels. Slit diaphragms are widened versions of the tight junctions and adhering junctions that link all contiguous epithelial cells together and are like miniature ladders. The pedicels form the sides of the ladder, and the slit diaphragms are the rungs. Spaces between slit diaphragms constitute the path through which the filtrate, once it has passed through the endothelial cells and basement membrane, travels to enter Bowman’s space.
Both the slit diaphragms and basement membrane are composed of an array of proteins, and while the basement membrane may contribute to the selectivity of the filtration barrier, integrity of the slit diaphragms is essential to prevent excessive leak of plasma protein (albumin). Some protein-wasting diseases are associated with abnormal slit diaphragm structure.
The selectivity of the filtration barrier is crucial for renal function. The barrier has to be leaky enough to permit free passage of everything that should be filtered, such as organic waste, yet restrictive to plasma proteins that should not be filtered. Selectivity of the barrier is based on both molecular size and electrical charge. Let us look first at size.