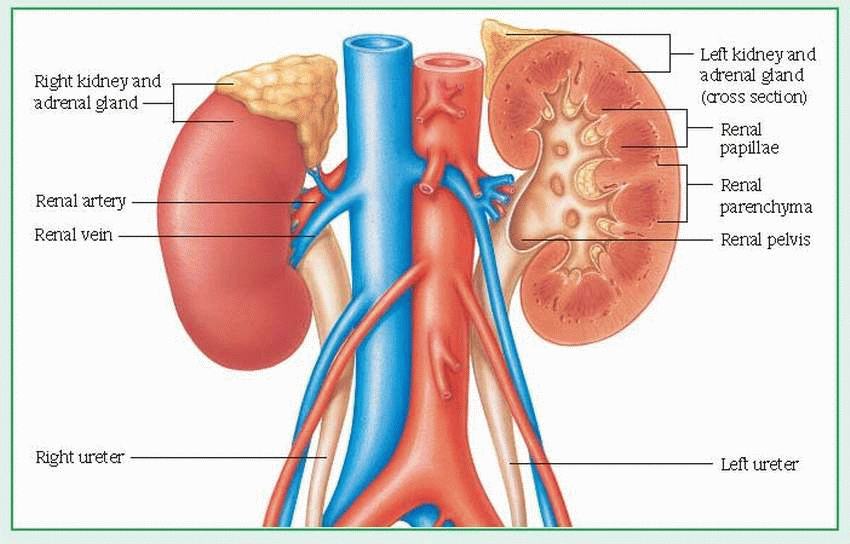
from the body through urine formation (by glomerular filtration, tubular reabsorption, and tubular secretion) and excretion. Glomerular filtration, the process of filtering the blood flowing through the kidneys, depends on the permeability of the capillary walls, vascular pressure, and filtration pressure. The normal glomerular filtration rate (GFR) is about 120 ml/minute.
If the tubules don’t reabsorb or secrete the substance, clearance equals the GFR.
If the tubules reabsorb it, clearance is less than the GFR.
If the tubules secrete it, clearance is greater than the GFR.
If the tubules reabsorb and secrete it, clearance is less than, equal to, or greater than the GFR.
the adrenal cortex. ADH alters the collecting tubules’ permeability to water. When plasma concentration of ADH is high, the tubules are very permeable to water, so a greater amount of water is reabsorbed, creating a high concentration but small volume of urine. The reverse is true if ADH concentration is low.
Intake and output assessment: Fluid intake and output measurement helps determine the patient’s hydration status but isn’t a reliable
method of evaluating renal function because urine output varies with different types of renal disorders. To provide the most useful and accurate information, use calibrated containers, establish baseline values for each patient, compare measurement patterns, and validate intake and output measurements by checking the patient’s weight daily. Monitor all fluid losses — including blood, vomitus, and diarrhea. Also assess wound and stoma drainage daily.
Specimen collection: Meticulous specimen collection is vital for valid laboratory data. If the patient is collecting the specimen, explain the importance of cleaning the meatal area thoroughly. The culture specimen should be caught midstream, in a sterile container; a specimen for urinalysis, in a clean container, preferably at the first voiding of the day. Begin a 24-hour specimen collection after discarding the first voiding; such specimens often necessitate special handling or preservatives. When obtaining a urine specimen from a catheterized patient, remember to avoid taking the specimen from the collection bag; instead, aspirate a sample through the collection port in the catheter, with a sterile needle and a syringe.
Kidney-ureter-bladder radiography: This test assesses size, shape, position, and areas of calcification of these organs.
Ultrasonography: This safe, painless procedure allows for visualization of the renal parenchyma, calyces, pelvis, ureters, and bladder. Because the test doesn’t depend on renal function, it’s useful in patients with renal failure and in detecting complications after kidney transplantation.
Invasive tests: See Invasive diagnostic tests for assessing the renal and urologic systems, pages 344 and 345.
Symptom | Possible cause |
Dribbling | Prostatic enlargement, strictures |
Dysuria | Infection, inflammation of bladder or urethra |
Edema | Nephrotic syndrome, failure |
Frequency | Infection, diabetes, bladder tumors, medications |
Hematuria | Glomerular diseases, trauma, neoplasms, renal calculi |
Hesitancy | Neurogenic bladder, infection |
Incontinence | Infection, neoplasms, prolapsed uterus |
Nocturia | Infection, nephrotic syndrome, diabetes, medications |
Oliguria | Failure, insufficiency, neoplasms |
Proteinuria | Glomerular diseases, infection |
Pyuria | Infection |
Renal colic | Calculi |
Urgency | Infection, prostatic disease, medications |
Normal serum value | Deviation | Normal urine value | Deviation | |
Sodium | 135 to 145 mEq/L | ↑ or N | 30 to 280 mEq/L | V |
Potassium | 3.8 to 5.5 mEq/L | ↑ | 25 to 100 mEq/L | ↓ |
Chloride | 100 to 108 mEq/L | ↑ | 110 to 250 mEq/24hr | ↓ |
Calcium | 8.9 to 10.1 mg/dl | ↓ | Female: < 250 mg/24 hr | ↓ |
Male: < 275 mg/24 hr | ||||
Phosphorus | 2.5 to 4.5 mg/dl | ↑or N | 1 g/24 hr | V |
Magnesium | 1.7 to 2.1 mEq/L | ↑ | < 150 mg/24 hr | ↓ |
Carbon dioxide combining power | 22 to 34 mEq/L | ↓ | ||
Specific gravity | 1.005 to 1.035 | ↓ | ||
pH | 4.5 to 8 | ↑ | ||
Blood urea nitrogen | 8 to 20 mg/dl | ↑ | 10 to 20 g/L | ↓ |
Creatinine | Female: 0.6 to 0.9 mg/dl | ↑ | Female: 0 to 80 mg/24 hr | ↓ |
Male: 0.8 to 1.2 mg/dl | Male: 0 to 40 mg/24 hr | |||
Osmolality | 280 to 295 mOsm/kg | V | 500 to 1,400 mOsm/kg | ↓ |
Uric acid | Female: 2.3 to 6 mg/dl | ↑ | ||
Male: 4.3 to 8 mg/dl | ||||
Glucose | 70 to 100 mg/dl | N | 0 | N |
Protein | 6.9 to 7.9 g/dl | ↓ or N | 0 | V |
Hematocrit | Female: 38% to 46% | ↓ | ||
Male: 42% to 54% | ||||
Hemoglobin | Female: 12 to 16 g/dl | ↓ | ||
Male: 14 to 18 g/dl | ||||
White blood cells | 4,000 to 10,000/l | None | V | |
Red blood cells | None | V | ||
Casts | None | V | ||
Bacteria | None | V | ||
Alkaline phosphatase | Female age 24 to 65: 82 to 282 U/L | ↑ | ||
Male age 19 or older: 98 to 251 U/L | ||||
KEY: ↑ = increased; ↓ = decreased; N = normal; V = varies | ||||
Procedure | Purpose | Special considerations |
Computed tomography scan (CT) or magnetic resonance imaging (MRI) | Although CT scan and MRI can be noninvasive, I.V. contrast material is often given to enhance the views obtained. These tests are especially helpful in evaluating renal or bladder mass lesions. | Before: Check for prior history of contrast sensitivity. After: After using I.V. contrast medium, observe for hypersensitivity reaction and hematoma at injection site. |
Cystoscopy | A fiber-optic scope is used to visualize the inside of the bladder in cystoscopy. | Before: Give sedatives as ordered. After: Offer increased fluids; administer analgesics; watch for hematuria and signs of perforation, hemorrhage, and infection (chills, fever, increased pulse rate, shock). |
Cystourethrography | In cystourethrography, X-rays and I.V. contrast material are used to determine size and shape of the bladder and urethra. | Before: Check for prior history of contrast sensitivity. During: Catheterize the patient. After: Offer increased fluids; observe for hypersensitivity reaction. |
Cystometry | Cystometry is used to evaluate bladder pressure, sensation, and capacity. A catheter is introduced into the bladder, and saline solution is instilled. Results are shown on a computer at the time of testing. | Before: Observe voiding; catheterize for residual urine. After: Remove catheter; watch for stress incontinence when patient coughs; watch voiding; catheterize for residual urine. |
Excretory urography | Excretory urography uses X-rays and I.V. contrast material to allow visualization of renal parenchyma, calyces, pelves, ureters, bladder, and stones. | Before: Check for prior history of contrast sensitivity. After: Observe for hypersensitivity reaction; watch for hematomas at injection site. |
Nephrotomography | In nephrotomography, I.V. contrast material and tomography are used to visualize parenchymia, calyces, and pelves in layers. | Before: Check for prior history of contrast sensitivity. After: Observe for hypersensitivity reaction. |
Renal angiography | Renal angiography uses contrast material injected into a catheter in the femoral artery or vein to allow visualization of the arterial tree and capillaries as well as venous drainage of the kidney. | Before: Check for prior history of contrast sensitivity. After: Observe for hypersensitivity reaction, hematomas and hemorrhage at injection site, and nephrotoxicity. Offer increased fluids. |
Renal scan | In renal scan, radioisotopes are administered I.V., and images of the kidney are taken at intervals to determine renal function. | Before: Check for prior history of sensitivity. After: Observe for hypersensitivity reaction. |
Renal biopsy | The specimen obtained in renal biopsy is used to develop histologic diagnosis and determine therapy and prognosis. | Before: Make sure the patient’s clotting times, prothrombin times, and platelet count are recorded on his chart. After: Apply gentle pressure to the bandage site. Watch for hemorrhage and hematoma at the biopsy site, and look for hematuria. |
Calcium oxalate calculi
Infection
of calcium oxalate stones, which lodge in the dilated cystic collecting ducts or pass through a ureter, and infection secondary to dilation of the ducts. These complications, which occur in about 30% of patients, are likely to produce severe colic, hematuria, lower urinary tract infection ([UTI]; burning on urination, urgency, frequency), and pyelonephritis. Secondary impairment of renal function from obstruction and infection occurs in only about 10% of patients.
To prevent infection, instruct the patient to bathe often and use proper toilet hygiene; this is especially important for a female patient because the proximity of the urinary meatus and the anus increases the risk of infection.
If infection occurs, stress the importance of completing the prescribed course of antibiotic therapy.
Emphasize the need for adequate fluid intake.
Explain all diagnostic procedures, and provide emotional support. Teach the patient how to collect a clean-catch urine specimen for culture. Check for allergy to excretory urography dye.
When the patient is hospitalized for a stone, strain all urine, administer analgesics as ordered, and force fluids. Before discharge, tell the patient to watch for and report any signs of stone passage and UTI.
Hypertension
Urinary tract infection (UTI)
Kidney infection
End-stage kidney disease
Kidney stones
Ruptured cysts
Excretory urography reveals enlarged kidneys, with elongation of pelvis, flattening of the calyces, and indentations caused by cysts. Excretory urography of the neonate shows poor excretion of contrast medium.
Ultrasound and computed tomography scan show kidney enlargement and the presence of cysts; tomography demonstrates multiple areas of cystic damage. Ultrasonography is the preferred imaging technique because it’s less expensive, doesn’t require contrast or radiation exposure, and is easily and safely performed on children and pregnant females.
Urinalysis and creatinine clearance tests are nonspecific tests that evaluate renal function and reveal urine protein or blood in the urine.
renal impairment progresses, selected patients may undergo dialysis, transplantation, or both. Cystic abscess or retroperitoneal bleeding may require surgical drainage; intractable pain (a rare symptom) may also require surgery. However, because this disease affects both kidneys, nephrectomy usually isn’t recommended because it increases the risk of infection in the remaining kidney.
Refer the young adult patient or the parents of infants with polycystic kidney disease for genetic counseling. Parents will probably have many questions about the risk to other offspring.
Provide supportive care to minimize any associated symptoms. Carefully assess the patient’s lifestyle and his physical and mental status; determine how rapidly the disease is progressing. Use this information to plan individualized patient care.
Acquaint yourself with all aspects of endstage renal disease, including dialysis and transplantation, so you can provide appropriate care and patient teaching as the disease progresses.
Explain all diagnostic procedures to the patient or to his family if the patient is an infant. Before beginning excretory urography or other procedures that use an iodine-based contrast medium, determine whether the patient has ever had an allergic reaction to iodine or shellfish. Even if the patient has no history of allergy, watch for an allergic reaction after performing the procedures.
Administer antibiotics as ordered for UTI. Stress to the patient the need to take the medication exactly as prescribed, even if symptoms are minimal or absent.
Volume overload
Pulmonary edema
Electrolyte imbalance
Metabolic acidosis
GI: anorexia, nausea, vomiting, diarrhea or constipation, stomatitis, bleeding, hematemesis, dry mucous membranes, uremic breath
Central nervous system (CNS): headache, drowsiness, irritability, confusion, peripheral neuropathy, seizures, coma
Cutaneous: dryness, pruritus, pallor, purpura and, rarely, uremic frost
Cardiovascular: early in the disease, hypotension; later, hypertension, arrhythmias, fluid
overload, heart failure, systemic edema, anemia, altered clotting mechanisms
Respiratory: pulmonary edema, Kussmaul’s respirations.
Measure and record intake and output, including all body fluids, such as wound drainage, nasogastric tube output, and diarrhea. Weigh the patient daily.
Assess Hb levels and HCT and replace blood components, as indicated. Don’t use whole blood if the patient is prone to heart failure and can’t tolerate extra fluid volume. Packed red blood cells deliver the necessary blood components without added volume.
Monitor vital signs. Watch for and report any signs of pericarditis (pleuritic chest pain, tachycardia, pericardial friction rub), inadequate renal perfusion (hypotension), and acidosis.
Maintain proper electrolyte balance. Strictly monitor potassium levels. Watch for symptoms of hyperkalemia (malaise, anorexia, paresthesia, or muscle weakness) and electrocardiogram changes (tall, peaked T waves, widening QRS segment, and disappearing P waves), and report them immediately. Avoid administering medications containing potassium.
Maintain nutritional status. Provide a highcalorie, low-protein, low-sodium, and lowpotassium diet, with vitamin supplements. Give the anorectic patient small, frequent meals.
Use sterile technique, because the patient with AKI is highly susceptible to infection. Personnel with upper respiratory tract infections shouldn’t provide care for the patient.
Prevent complications of immobility by encouraging frequent coughing and deep breathing and by performing passive range-of-motion exercises. Help the patient walk as soon as possible.
Provide good mouth care frequently because mucous membranes are dry. If stomatitis occurs, an antibiotic solution may be ordered. Have the patient swish the solution around in his mouth before swallowing.
Monitor for GI bleeding by guaiac testing all stools for blood. Administer medications carefully, especially antacids and stool softeners. Use aluminum-hydroxide-based antacids; magnesium-based antacids can cause serum magnesium levels to rise to critical levels.
Use appropriate safety measures, such as side rails and restraints, because the patient with CNS involvement may be dizzy or confused.
Provide emotional support to the patient and his family. Reassure them by clearly explaining all procedures.
If the patient requires hemodialysis, check the blood access site (arteriovenous fistula, subclavian or femoral catheter) as per facility protocol or every 2 hours for patency and signs of clotting. Don’t use the arm with the shunt or fistula for taking blood pressures or drawing blood. Weigh the patient before beginning dialysis. During dialysis, monitor vital signs, clotting times, blood flow, the function of the vascular access site, and arterial and venous pressures. Watch for complications, such as septicemia, embolism, hepatitis, and rapid fluid and electrolyte loss. After dialysis, monitor vital signs and the vascular access site; weigh the patient; watch for signs of fluid and electrolyte imbalances.
During peritoneal dialysis, position the patient carefully. Elevate the head of the bed to reduce pressure on the diaphragm and aid respiration. Be alert for signs of infection (cloudy drainage, elevated temperature) and, rarely, bleeding. If pain occurs, reduce the amount of dialysate. Monitor the diabetic patient’s blood glucose periodically, and administer insulin as ordered. Watch for complications, such as peritonitis, atelectasis, hypokalemia, pneumonia, and shock.
Use standard precautions when handling all blood and body fluids.
Sexually active women: Intercourse increases the risk of bacterial contamination.
Pregnant women: About 5% develop asymptomatic bacteriuria; if untreated, about 40% develop pyelonephritis.
Diabetics: Neurogenic bladder causes incomplete emptying and urinary stasis; glycosuria may support bacterial growth in the urine.
Persons with other renal diseases: Compromised renal function aggravates susceptibility.
Recurrence of pyelonephritis
Sepsis
Perinephric abscess
Renal failure
infection is likely and may cause symptoms to recur later.
Pyuria (pus in urine): Urine sediment reveals the presence of leukocytes singly, in clumps, and in casts; and, possibly, a few red blood cells.
Significant bacteriuria: Urine culture reveals more than 100,000 organisms/µl of urine.
Low specific gravity and osmolality: These findings result from a temporarily decreased ability to concentrate urine.
Slightly alkaline urine pH.
Proteinuria, glycosuria, and ketonuria: These conditions are less common.
Costovertebral angle tenderness Excretory urography or computed tomography scan of the kidneys, ureters, and bladder also help in the evaluation of acute pyelonephritis by revealing calculi, tumors, or cysts in the kidneys and the urinary tract. In addition, excretory urography may show asymmetrical kidneys.
Administer antipyretics for fever.
Encourage fluids to achieve urine output of more than 2,000 ml/day. This helps to empty the bladder of contaminated urine. Don’t encourage intake of more than 2 to 3 qt (2 to 3 L) because this may decrease the effectiveness of the antibiotics.
Provide an acid-ash diet to prevent stone formation.
Teach proper technique for collecting a clean-catch urine specimen. Be sure to refrigerate or culture a urine specimen within 30 minutes of collection to prevent overgrowth of bacteria.
Stress the need to complete prescribed antibiotic therapy, even after symptoms subside. Encourage long-term follow-up care for highrisk patients.
Observe strict sterile technique during catheter insertion and care.
Instruct women to prevent bacterial contamination by wiping the perineum from front to back after defecation.
Advise routine checkups for patients with a history of urinary tract infections. Teach them to recognize signs of infection, such as cloudy urine, burning on urination, urgency, and frequency, especially when accompanied by a low-grade fever.
Acute kidney injury or chronic kidney failure
End-stage renal disease
Hypertension
Heart failure
Pulmonary edema
Chronic glomerulonephritis
Nephrotic syndrome
Check vital signs and electrolyte values. Monitor intake and output and daily weight. Assess renal function daily through serum creatinine, blood urea nitrogen, and urine creatinine clearance levels. Watch for and immediately
report signs of acute renal failure (oliguria, azotemia, and acidosis).
Consult the dietitian to provide a diet high in calories and low in protein, sodium, potassium, and fluids.
Protect the debilitated patient against secondary infection by providing good nutrition, using good hygienic technique, and preventing contact with infected persons.
Bed rest is necessary during the acute phase. Allow the patient to gradually resume normal activities as symptoms subside.
Provide emotional support for the patient and his family. If the patient is on dialysis, explain the procedure fully.
Advise the patient with a history of chronic upper respiratory tract infections to immediately report signs of infection (fever, sore throat).
Tell the patient that follow-up examinations are necessary to detect chronic renal failure. Stress the need for regular blood pressure, urinary protein, and renal function assessments during the convalescent months to detect recurrence. After APSGN, gross hematuria may recur during nonspecific viral infections; abnormal urinary findings may persist for years.
Encourage pregnant women with a history of APSGN to have frequent medical evaluations because pregnancy further stresses the kidneys and increases the risk of chronic renal failure.
diseased tubular epithelium that allows leakage of glomerular filtrate across the membranes and reabsorption of filtrate into the blood
obstruction of urine flow by the collection of damaged cells, casts, red blood cells (RBCs), and other cellular debris within the tubular walls
ischemic injury to glomerular epithelial cells, resulting in cellular collapse and decreased glomerular capillary permeability
ischemic injury to vascular endothelium, eventually resulting in cellular swelling and obstruction.
Fluid and electrolyte imbalance
Heart failure
GI hemorrhage
Pulmonary edema
of ATN. (See A close look at acute tubular necrosis.) The first recognizable effect may be decreased urine output. Generally, hyperkalemia and the characteristic uremic syndrome soon follow, with oliguria (or, rarely, anuria) and confusion, which may progress to uremic coma. Other possible complications may include heart failure, uremic pericarditis, pulmonary edema, uremic lung, anemia, anorexia, intractable vomiting, and poor wound healing due to debilitation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree