, Arthur H. Cohen2, Robert B. Colvin3, J. Charles Jennette4 and Charles E. Alpers5
(1)
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
(2)
Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA
(3)
Department of Pathology Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA
(4)
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
(5)
Department of Pathology, University of Washington, Seattle, Washington, USA
Abstract
Each kidney weighs approximately 150 g in adults, with ranges of 125–175 g for men and 115–155 g for women; both together represent 0.4 % of the total body weight. Each kidney is supplied by a single renal artery originating from the abdominal aorta; the main renal artery branches to form anterior and posterior divisions at the hilus and divides further, its branches penetrating the renal substance proper as interlobar arteries, which course between lobes. Interlobar arteries extend to the corticomedullary junction and give rise to arcuate arteries, which arch between cortex and medulla and course roughly perpendicular to interlobar arteries. Interlobular arteries, branches of arcuate arteries, run perpendicular to the arcuate arteries and extend through the cortex toward the capsule (Fig. 1.1). Afferent arterioles branch from the interlobular arteries and give rise to glomerular capillaries (Fig. 1.2). A glomerulus represents a spherical bag of capillary loops arranged in several lobules (Fig. 1.3); the capillaries merge to exit the glomerulus as efferent arterioles, which, in most nephrons, branch to form another vascular bed, peritubular or interstitial capillaries, which surround tubules. Efferent arterioles from juxtamedullary glomeruli extend into the medulla as vasa recta, which supply the outer and inner medulla. The vasa recta and peritubular capillaries collect, forming into interlobular veins; the veins follow the arteries in distribution, size, and course and leave the kidneys as renal veins, which empty into the inferior vena cava.
Normal Anatomy
Each kidney weighs approximately 150 g in adults, with ranges of 125–175 g for men and 115–155 g for women; both together represent 0.4 % of the total body weight. Each kidney is supplied by a single renal artery originating from the abdominal aorta; the main renal artery branches to form anterior and posterior divisions at the hilus and divides further, its branches penetrating the renal substance proper as interlobar arteries, which course between lobes. Interlobar arteries extend to the corticomedullary junction and give rise to arcuate arteries, which arch between cortex and medulla and course roughly perpendicular to interlobar arteries. Interlobular arteries, branches of arcuate arteries, run perpendicular to the arcuate arteries and extend through the cortex toward the capsule (Fig. 1.1). Afferent arterioles branch from the interlobular arteries and give rise to glomerular capillaries (Fig. 1.2). A glomerulus represents a spherical bag of capillary loops arranged in several lobules (Fig. 1.3); the capillaries merge to exit the glomerulus as efferent arterioles, which, in most nephrons, branch to form another vascular bed, peritubular or interstitial capillaries, which surround tubules. Efferent arterioles from juxtamedullary glomeruli extend into the medulla as vasa recta, which supply the outer and inner medulla. The vasa recta and peritubular capillaries collect, forming into interlobular veins; the veins follow the arteries in distribution, size, and course and leave the kidneys as renal veins, which empty into the inferior vena cava.
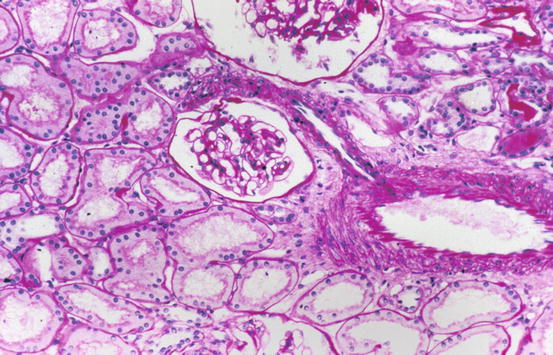
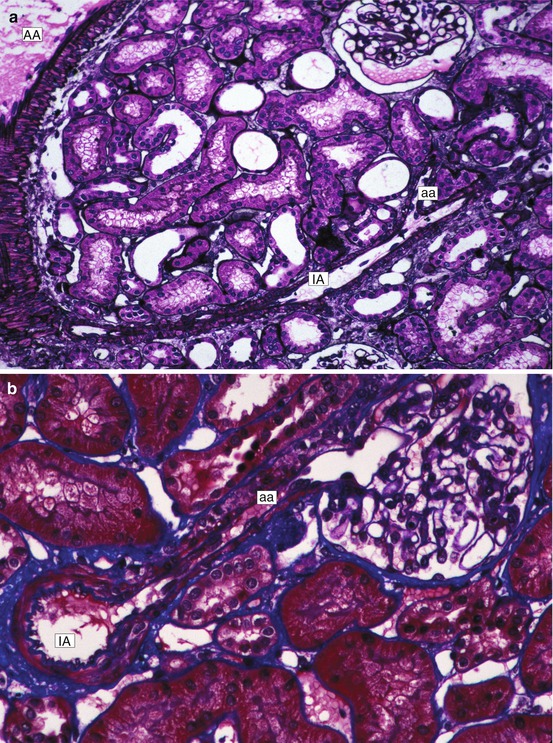
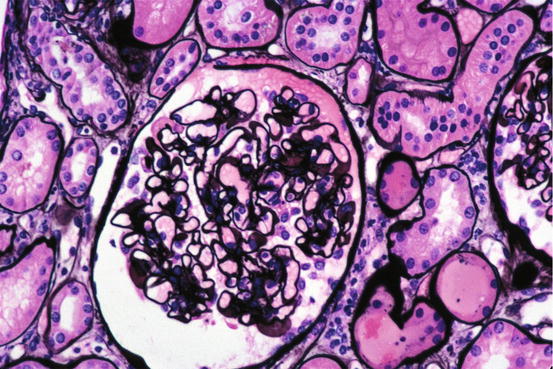

Fig. 1.1
Low magnification of cortex with portions of two glomeruli, tubules, and interstitium and interlobular artery with arteriolar branch [periodic acid-Schiff (PAS) stain]

Fig. 1.2
(a) Low magnification of cortex. An arcuate artery (AA), interlobular artery (IA), and afferent arteriole (aa) are in continuity (Jones silver stain). (b) Interlobular artery (IA) with afferent arteriole (aa) extending into glomerulus (Masson trichrome stain)

Fig. 1.3
Normal glomerulus with surrounding normal tubules and interstitium (Jones silver stain)
The kidneys have three major components: the cortex, the medulla, and the collecting system. On the cut surface, the cortex is the pale outer region, approximately 1.5 cm in thickness, which has a granular appearance because of the presence of glomeruli and convoluted tubules. The medulla, a series of pyramidal structures with apical papillae, numbers normally 8–18 and has a striped or striated appearance because of the parallel arrangement of the tubular structures. The bases of the pyramids are at the corticomedullary junction and the apices extend into the collecting system. Cortical parenchyma extends into spaces between adjacent pyramids; this portion of the cortex is known as the columns of Bertin. A medullary pyramid with surrounding cortical parenchyma, which includes both columns of Bertin and the subcapsular cortex, constitutes a renal lobe. The collecting system consists of the pelvis, which represents the expanded upper portion of the ureter, and is more or less funnel shaped. Each pelvis has two or three major branches known as the major calyces. Each calyx divides further into three or four smaller branches known as minor calyces, each usually receiving one medullary papilla.
Each kidney contains approximately one million nephrons, each composed of a glomerulus and attached tubules. Glomeruli are spherical collections of interconnected capillaries within a space (Bowman’s space) lined by flattened parietal epithelial cells (Fig. 1.3). Bowman’s space is continuous with the tubules, with the orifice of the proximal tubule generally at the pole opposite the glomerular hilus, where the afferent and efferent arterioles enter and leave, respectively. A layer of visceral epithelial cells, also called podocytes, covers the outer aspects of the glomerular capillaries. Each podocyte has a large body containing the nucleus and cytoplasmic extensions, which divide, forming small fingerlike processes that interdigitate with similar structures from adjacent cells and cover the capillaries. These interdigitating processes, known as pedicles, are also called foot processes because of their appearance on transmission electron microscopy. The space between adjacent foot processes is known as the filtration slit; adjacent foot processes are joined together by a thin membrane known as the slit-pore diaphragm. The slit diagram is composed of a complex of the transmembrane proteins nephrin, NEPH1 through NEPH3, podocin, Fat1, VE-cadherin, and P-cadherin. Mutations in NEPH1 and podocin cause proteinuria. Epithelial cells cover the glomerular capillary basement membrane, a three-layer structure with a central thick layer slightly electron-dense (lamina densa) and thinner electron-lucent layers beneath epithelial and endothelial cells (lamina rara externa and lamina rara interna, respectively) (Fig. 1.4). The glomerular basement membrane is composed predominately of type IV collagen with six distinct λ chains, laminin 11, entactin, and sulfated proteoglycans. The glomerular basement membrane in adults measures approximately 340–360 nanometers (nm) in thickness and is significantly thicker in men than in women. The endothelial cells are thin and have multiple fenestrae, each measuring approximately 80 nm in diameter. The surface of endothelial cells is negatively charged, with a surface coat glycocalyx composed of anionic glycosaminoglycans and glycoproteins. The capillary tufts are supported by the mesangium, which represents the intraglomerular continuation of the arteriolar walls. The mesangium has two components. The extracellular one, mesangial matrix, has many structural, compositional, and, therefore, tinctorial properties similar to basement membrane. The cells of the mesangium are known as mesangial cells, of which there are two types: modified smooth muscle cells, representing greater than 95 % of the cellular population, and bone marrow-derived cells, representing the remainder. Mesangial cells have numerous functions including contraction, production of extracellular matrix, secretion of inflammatory and other active mediators, phagocytosis, and migration from the central zone where they are normally situated (Fig. 1.5).
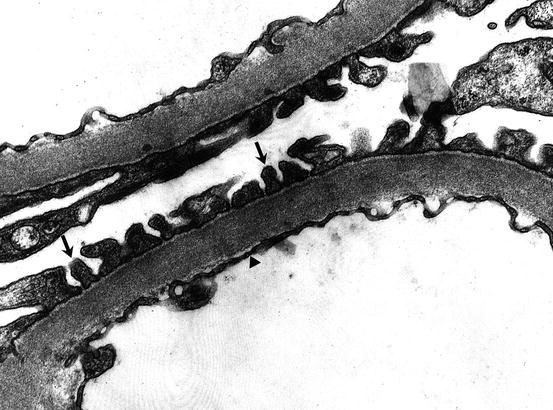
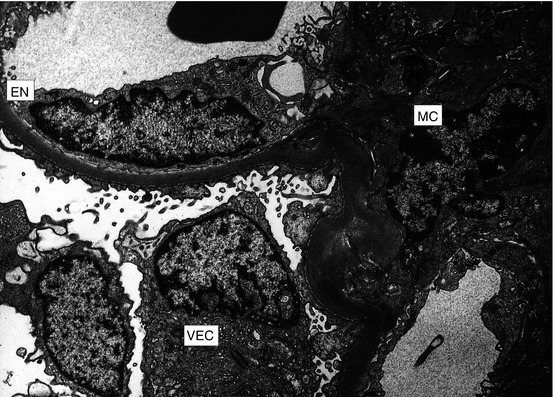

Fig. 1.4
Portion of glomerular capillary wall by electron microscopy. Individual foot processes of podocytes (arrows) cover the basement membrane and endothelial cell cytoplasm (arrowhead) lines the lumen

Fig. 1.5
Portion of glomerulus indicating different cell types: capillary endothelial cell (EN), visceral epithelial cell (VEC), and mesangial cell (MC) (electron microscopy)
The proteoglycans of the glomerular basement membrane are negatively charged; similarly, the surface of both epithelial and endothelial cells is anionically charged because of sialoglycoproteins in the cellular coats. Both of these negatively charged structures are responsible for the charge–selective barrier to filtration of capillary contents. The basement membrane, which, along with the fenestrated endothelial cell, allows for ready filtration of water and small substances, is known as the size–selective barrier. The podocyte in the adult is responsible for the production and maintenance of basement membrane.
The remaining portion of the nephron is divided into proximal tubules, which are often convoluted; the loop of Henle, with both descending and ascending limbs; and the distal tubule. The proximal tubular cells have well-developed closely packed microvillus luminal surfaces known as the brush border. The cells are larger than those of the distal tubules, which have relatively few surface microvilli. Each tubule is surrounded completely by a basement membrane. Adjacent tubular basement membranes are in almost direct contact with one another and separated by a small amount of connective tissue known as the interstitium, which contain peritubular capillaries (Fig. 1.6). At the vascular pole of the glomerulus and the site of entrance of the afferent arteriole, the cells of the arteriolar wall are modified into secretory cells known as juxtaglomerular cells; these produce and secrete renin, contained in granules. The macula densa, a portion of the distal tubule at the glomerular hilus, is characterized by smaller and more crowded distal tubular cells, which are in contact with the juxtaglomerular cells. Surrounding the macula densa and afferent arteriole are lacis cells, which are mesenchymal cells similar to mesangial cells.
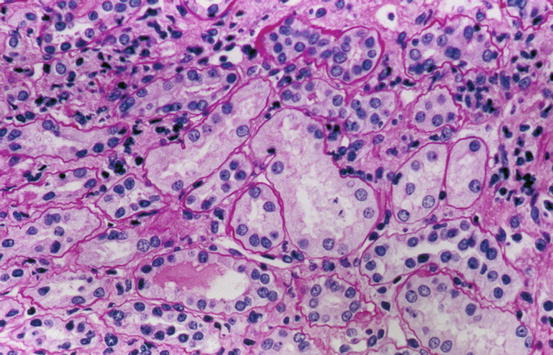

Fig. 1.6
Normal cortical tubules, interstitium, and peritubular capillaries; most of the tubules are proximal, with well-defined brush borders (PAS stain)
Examination of Renal Tissue
Because of the types of diseases and the renal components that are abnormal, the preparation of tissue specimens for examination is somewhat complex considering the required methods of study. These include sophisticated light microscopy, immunofluorescence, and electron microscopy. For light microscopy, the elucidation of lesions of glomeruli mandates that a variety of histochemical stains be used and that tissue sections be cut thinner than for other tissues. Furthermore, to take best advantage of the stains, many investigators and renal pathologists have found that formalin, Zenker’s solution, or many of the more commonly used fixatives result in substandard preparations. Consequently, alcoholic Bouin’s solution (Duboscq-Brasil) is the fixative of choice for superb morphology and stains. However, methods for many immunostains and molecular studies are based on formalin-fixed tissue and results are unreliable. Consequently, there has been a steady shift away from Bouin’s and toward formalin in recent years. For the elucidation of glomerular structure and pathology, it is necessary that the extracellular matrix components (basement membrane, mesangial matrix) be preferentially stained. Table 1.1 indicates staining characteristic of normal and abnormal renal structures. In paraffin-embedded sections, the hematoxylin and eosin stain does not ordinarily allow for distinction of extracellular matrix from cytoplasm in a clear or convincing manner. Periodic acid-Schiff (PAS), periodic acid-methenamine silver (Jones), and Masson’s trichrome stains all provide excellent definition of extracellular material. Each stain has its advantages and disadvantages, and, as a rule, all are used in evaluating renal tissues especially biopsies. The PAS reagent stains glomerular basement membranes, mesangial matrix, and tubular basement membranes red (positive), while the Jones stain (periodic acid-methenamine silver) colors the same components black, providing clear contrast between positively and negatively staining structures. Masson’s trichrome colors extracellular glomerular matrix (capillary basement membranes, mesangial matrix) and tubular basement membranes blue, clearly distinguished from cells and abnormal material that accumulates in pathologic circumstances. Congo red, elastic tissue, and other stains are employed when indicated. The tissue sections should be no greater than 2–3 μm in thickness, for the definition of glomerular pathology, especially regarding cellularity, is dependent on sections of this thickness. The ability to detect subtle pathologic abnormalities is enhanced with thinner sections. Especially for glomerular diseases, immunohistochemistry is necessary for evaluation of renal tissues, especially for diagnosing glomerular diseases. Most laboratories utilize immunofluorescence for identifying and localizing immunoglobulins, complement, fibrin, and other immune substances within renal tissues; fluorescein-labeled antibodies to the following are used: immunoglobulin G (IgG), IgA, IgM, C1q, C3, albumin, fibrin, and kappa and lambda immunoglobulin light chains. For transplant biopsies, antibody to C4d is routinely utilized. Fluorescence positivity in glomeruli as well as tubular basement membranes is described as granular or linear (Fig. 1.7). Regardless of the immunopathologic mechanisms responsible for the granular deposits, there is an electron microscopic counterpart to granular deposits; by electron microscopy, extracellular masses of electron-dense material correspond to the deposits. The granular deposits can be appreciated in tissue prepared for light microscopy; this is best demonstrated and documented with the use of Masson’s trichrome stain, where granular deposits appear as bright fuchsinophilic (orange, red orange) smooth homogeneous structures. There is no regular ultrastructural or light microscopic counterpart to linear staining (Table 1.2). Electron microscopy is routinely utilized in the study of renal tissues. For glomerular and some tubulointerstitial diseases, this method is mandatory and helps localize deposits, detects extremely small deposits, and documents alterations of cellular and basement membrane structure. Immunofluorescence and electron microscopy are also often necessary and helpful in diagnosing other tubular, interstitial, and vascular lesions.
Table 1.1




Staining characteristics of selected normal and abnormal renal structures
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


