Objectives
- Describe how the excretion of salt and water serves the needs of the cardiovascular system.
- Name the major regulators of sodium excretion.
- Describe the systemic renin-angiotensin-aldosterone system and where its components are formed.
- State the major actions of angiotensin II.
- State the major actions of aldosterone.
- Describe the 3 major regulators of renin secretion.
- Describe the functions of the macula densa.
- Define tubuloglomerular feedback and describe the mechanism for tubuloglomerular feedback and autoregulation of glomerular filtration rate.
- State the origin of atrial natriuretic peptides, the stimulus for their secretion, and their effect on sodium reabsorption and glomerular filtration rate.
- State the 2 urinary factors that determine water excretion.
- Describe the origin of antidiuretic hormone and the major controls of its secretion.
The Goals of Regulation
Sodium and water excretion are regulated by an array of control systems. Seemingly every hormone, cytokine, sympathetic transmitter, and paracrine agent exerts an influence somewhere in the kidney. Given this complexity it might seem impossible to present a meaningful description of how sodium and water excretion are regulated. Certainly we cannot begin with each of the known regulated processes and predict their behavior in every situation. However, if we look at the goals of regulation—what regulation accomplishes—we can fit the various regulated processes into a framework of cooperative actions that serve the needs of the body.1
![]() The overriding goals of regulating sodium and water excretion are to support the requirements of the cardiovascular (CV) system. This is manifested in 2 ways: (1) the kidneys maintain a sufficient ECF volume to fill the vascular space and (2) keep the osmolality of the ECF at a level consistent with cellular health. As explained in more detail in the next section, the kidneys and the CV system work cooperatively to ensure that peripheral tissue is sufficiently perfused. An adequate circulating volume is one of the essential requirements for tissue perfusion and it is the kidneys that control this volume. Osmolality is the ratio of solute content to water content. Sodium and chloride together account for 80% of the normal extracellular solute; thus the excretion of sodium and water by the kidneys regulates osmolality in the tight range that is needed for the health of tissue cells. These goals are another way of looking at the concept of balance that we described earlier. When the kidneys alter excretion of sodium and water to preserve proper amounts in the body, they are maintaining balance.
The overriding goals of regulating sodium and water excretion are to support the requirements of the cardiovascular (CV) system. This is manifested in 2 ways: (1) the kidneys maintain a sufficient ECF volume to fill the vascular space and (2) keep the osmolality of the ECF at a level consistent with cellular health. As explained in more detail in the next section, the kidneys and the CV system work cooperatively to ensure that peripheral tissue is sufficiently perfused. An adequate circulating volume is one of the essential requirements for tissue perfusion and it is the kidneys that control this volume. Osmolality is the ratio of solute content to water content. Sodium and chloride together account for 80% of the normal extracellular solute; thus the excretion of sodium and water by the kidneys regulates osmolality in the tight range that is needed for the health of tissue cells. These goals are another way of looking at the concept of balance that we described earlier. When the kidneys alter excretion of sodium and water to preserve proper amounts in the body, they are maintaining balance.
There is a separate goal of regulation that differs from those stated above. Variations in renal blood flow (RBF) and glomerular filtration rate (GFR) are major means of regulating sodium excretion. However, the kidneys cannot change blood flow and filtration to such extreme values that they compromise the metabolic health of the kidneys or interfere with the excretion of substances other than sodium, particularly organic waste. Thus, another goal is to limit the sodium-related changes in RBF and GFR that might otherwise reach deleterious levels.
1A characteristic of most physiological control systems is redundancy. In cases of renal pathology, experimental ablation studies or genetic knockout of specific components, parallel controls that may play a minor role in normal circumstances can be upregulated to compensate for defective or missing elements.
Sodium Excretion: The Cardiovascular Connection
The kidneys work in partnership with the CV system. The CV system generates the pressure necessary for glomerular filtration and drives the high flow needed to maintain a stable cortical interstitial solute composition. In turn, the kidneys maintain blood volume, regulate plasma osmolality, and secrete mediators that affect both cardiac performance and vascular tone (Figure 7–1).
Figure 7–1.
Influence of the kidneys on the CV system. The kidneys affect blood volume via their production of erythropoietin, which stimulates red blood cell production, and via their control of salt and water excretion. They also influence total peripheral resistance via the actions on angiotensin II (see text for details). The combination of blood volume and total peripheral resistance affects blood pressure.
The kidneys partner with the CV system. The CV system provides the high pressure needed to drive glomerular filtration; the kidneys maintain the blood volume necessary to fill the vascular tree. |
Blood is composed primarily of red blood cells (approximately 45%) and blood plasma (approximately 55%). The kidneys are crucial for both parts—they secrete the hormone erythropoietin, which stimulates production of red blood cells, and they regulate the extracellular fluid (ECF) volume, of which blood plasma is a significant part (see Figure 6–1). Although not exact, there is an approximate proportionality between blood volume and total ECF volume. Blood volume tends to increase and decrease as ECF volume increases and decreases due to shifts of fluid between the plasma and tissue interstitial space. Thus, maintenance of blood volume is largely a matter of maintaining ECF volume.
![]() It is obvious why water is crucial for ECF volume, but why is sodium so important? The answer stems from 2 facts. First, ECF osmolality is tightly regulated, and second, the osmotic content of the ECF depends critically on its sodium content. We can approximate ECF osmolality as follows:
It is obvious why water is crucial for ECF volume, but why is sodium so important? The answer stems from 2 facts. First, ECF osmolality is tightly regulated, and second, the osmotic content of the ECF depends critically on its sodium content. We can approximate ECF osmolality as follows:
Furthermore, since almost all of the ECF solute is accounted for by sodium and an equivalent number of anions (mostly chloride and bicarbonate), the amount of ECF solute is approximately twice the sodium content. We can write the previous expression as:
Therefore, in the face of tightly controlled ECF osmolality (see later discussion), ECF volume varies directly with sodium content.
This raises a question—how do the kidneys know how much sodium there is in the ECF; in other words, how do they know whether to increase or minimize sodium excretion? There is no mechanism for the body to assay sodium content per se. Instead the detection of sodium content is indirect, based on a combination of assessing sodium concentration and vascular pressures. Glial cells in regions of the brain called the circumventricular organs (described later in this chapter) have sensory channels (Nax) that respond to and act as detectors of extracellular sodium concentration. The glial cells modulate the activity of nearby neurons involved in the control of body sodium. There are also neurons in the hypothalamus containing Nax channels that respond to the sodium concentration in the cerebrospinal fluid. Thus cells in or near the hypothalamus monitor extracellular sodium concentration.
As described above, ECF volume varies with sodium content at any given concentration. Volume affects pressures in different regions of the vasculature. Because vascular pressures are so important in regard to sodium excretion we will briefly review CV pressure regulation before describing its role in sodium excretion.
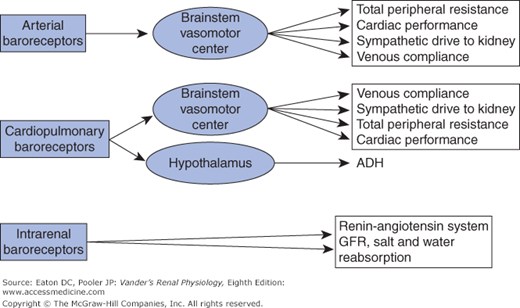
![]() Vascular pressures are assessed by baroreceptors—cells that deform in response to changes in local intravascular pressure. Three sets of baroreceptors are important for controlling sodium excretion (Figure 7–2). These are (1) arterial baroreceptors, nerve cells that mediate the classic baroreceptor reflex, (2) cardiopulmonary baroreceptors that are also nerve cells and work in parallel with the arterial baroreceptors, and (3) intrarenal baroreceptors, which are not nerve cells. We will describe their operation shortly. Arterial baroreceptors are located in the carotid arteries and arch of the aorta and sense pressure on a beat-by-beat basis. The cardiopulmonary baroreceptors have sensory endings located in the cardiac atria and parts of the pulmonary vasculature. They, like the arterial baroreceptors, send afferent neural information to the central nervous system. They are often called low-pressure baroreceptors because they assess pressures in regions of the vascular tree where pressures are much lower than in the arteries. The cardiopulmonary baroreceptors serve as de facto blood volume detectors in the sense that pressures in the atria and pulmonary vessels rise when blood volume increases and fall when blood volume decreases.
Vascular pressures are assessed by baroreceptors—cells that deform in response to changes in local intravascular pressure. Three sets of baroreceptors are important for controlling sodium excretion (Figure 7–2). These are (1) arterial baroreceptors, nerve cells that mediate the classic baroreceptor reflex, (2) cardiopulmonary baroreceptors that are also nerve cells and work in parallel with the arterial baroreceptors, and (3) intrarenal baroreceptors, which are not nerve cells. We will describe their operation shortly. Arterial baroreceptors are located in the carotid arteries and arch of the aorta and sense pressure on a beat-by-beat basis. The cardiopulmonary baroreceptors have sensory endings located in the cardiac atria and parts of the pulmonary vasculature. They, like the arterial baroreceptors, send afferent neural information to the central nervous system. They are often called low-pressure baroreceptors because they assess pressures in regions of the vascular tree where pressures are much lower than in the arteries. The cardiopulmonary baroreceptors serve as de facto blood volume detectors in the sense that pressures in the atria and pulmonary vessels rise when blood volume increases and fall when blood volume decreases.
Figure 7–2.
Baroreceptors and the major processes they influence. Arterial baroreceptors sense pressures in the aorta and carotid arteries and send afferent information to the brainstem vasomotor center, which then regulates CV and renal processes via autonomic efferents. Cardiopulmonary baroreceptors sense pressure in the cardiac atria and pulmonary arteries, thereby being responsive to the filling of the vascular tree. They send afferent information in parallel with the arterial baroreceptors. Although there is overlap between the influences of the 2 sets of baroreceptors, the cardiopulmonary baroreceptors have a particularly important influence on the hypothalamus, which regulates secretion of ADH. The intrarenal baroreceptors have a major role in the renin-angiotensin system (see text for details).
The information from neural baroreceptors is directed to a control center for vascular pressures consisting of nuclei in the medulla oblongata (lower region of the brainstem close to where the brainstem merges with the spinal cord). Collectively these nuclei are called the vasomotor center. The vasomotor center stimulates vascular tone (vasoconstriction) throughout the body via the sympathetic nervous system. In the arterioles of the peripheral vasculature this sympathetic tone maintains total peripheral resistance, and in the peripheral venous system it maintains central venous pressure via its ability to lower the compliance of large veins. By altering venous constriction in different vascular beds the vasomotor center can shift blood volume between different organs. The vasomotor center also sends both sympathetic stimulatory and parasympathetic inhibitory signals to the heart.
Arterial baroreceptors exert tonic inhibition of the medullary vasomotor center, resulting in a brake on sympathetic drive. An increase in arterial pressure causes greater firing of the baroreceptors, more inhibition and therefore less sympathetic drive to the periphery, whereas a decrease in arterial pressure reduces firing in the baroreceptors and less inhibition, allowing more sympathetic drive. The resulting changes in vascular tone alter total peripheral resistance and help stabilize arterial pressure. The cardiopulmonary baroreceptors act in the same way, but respond, not to arterial pressure, but rather to pressures in the cardiac atria and pulmonary vessels.
The kidneys, being part of the peripheral vasculature, respond to changes in sympathetic drive and contribute to changes in total peripheral resistance. However, as we will describe later, changes in sodium excretion in response to sympathetic drive are even more important than the renal contribution to total peripheral resistance.
The amount of sympathetic drive on the heart, arterioles, and large veins changes very rapidly when pressure begins to change as a result of muscle activity or simple changes in posture. The result is to stabilize arterial pressure at its set point, the mean arterial pressure, which for most people is slightly less than 100 mm Hg. The set point is not rigidly fixed, however; it varies during the day, depending on activity and levels of excitement, and decreases approximately 20% during sleep.2
The baroreceptor/sympathetic drive system described above is very effective in maintaining arterial pressure at the set point. A major complication and area of uncertainty lies in the long-term control of blood pressure. The question of “what sets the set point” cannot be answered with confidence.3 However, it is clear that malfunction in the renal handling of sodium or malfunction in renal signaling can lead to hypertension. Any consideration of the long-term control of blood pressure must include renal actions.
2As an example of this variation, some patients experience “white coat hypertension,” an elevation of blood pressure that is manifested in response to the stress of being in a doctor’s office.
3Arterial pressure is one among many physiological variables regulated around a set point, for example, body temperature, partial pressure of carbon dioxide, or plasma concentration of glucose. Although much is known about the mechanisms that keep these variables close to their set points, the question of “what sets their set points” remains unclear.
Major Controllers of Sodium Excretion
![]() Many signals exert control over sodium excretion. Given the preceding discussion, it should be clear that most signals directly or indirectly relate to events in the CV system. The major cardiovascular-related signaling systems are the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS).
Many signals exert control over sodium excretion. Given the preceding discussion, it should be clear that most signals directly or indirectly relate to events in the CV system. The major cardiovascular-related signaling systems are the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS).
The sympathetic nervous system is capable of an emergency “fight-or-flight” response, but its normal operation is a differential modulation of various functions within different organs. The vasculature and tubules of the kidney are innervated by postganglionic sympathetic neurons that release norepinephrine. In most regions of the kidney, norepinephrine is recognized by α-adrenergic receptors. In the renal vasculature activation of α1-adrenergic receptors causes vasoconstriction of afferent and efferent arterioles. This reduces RBF and GFR.
Quite obviously, GFR is a crucial determinant of sodium excretion. Without filtration there is no excretion. However, except in body emergencies such as hypovolemic shock, GFR is kept within rather narrow limits due to autoregulatory processes detailed later. Thus although neural control does affect GFR, this component of sympathetic control is probably less important in normal circumstances than its effect on sodium reabsorption. Neural control of the renal vasculature is exerted primarily on blood flow in the cortex, allowing preservation of medullary perfusion even when cortical blood flow is reduced.
The proximal tubule epithelial cells are innervated by α1– and α2-adrenergic receptors. Stimulation of these receptors in the proximal tubule by norepinephrine activates both components of the main transcellular sodium reabsorptive pathway, that is, the sodium–hydrogen antiporter NHE3 in the apical membrane and the Na-K-ATPase in the basolateral membrane. This pathway is activated in situations in which it is appropriate to reduce sodium excretion, for example, volume depletion or low blood pressure.
Sympathetic stimulation stimulates sodium reabsorption in the proximal tubule and reduces renal blood flow and GFR. |
The effects of sympathetic stimulation on cells in the distal nephron are less straightforward. Other agonists are coreleased with norepinephrine and affect other receptor types (eg, ATP acting on purinergic receptors). However, the overall outcome of sympathetic stimulation of the kidney is clearly reduced sodium excretion.
Major Controllers of Sodium Excretion
Even more important to the control of sodium excretion than sympathetic input by itself is the renin-angiotensin system. We will soon see that the renin-angiotensin system and the sympathetic nervous system are interacting systems and cannot be viewed as separate controllers. What is traditionally described as the renin-angiotensin system is really a set of renin-angiotensin systems. There is both a systemic circulating renin-angiotensin system, and separate organ specific renin-angiotensin systems in many tissues, including, but not limited to, the heart, sex organs, brain, and the kidneys. In addition, the circulating renin-angiotensin system is intimately involved in controlling the steroid hormone aldosterone. The circulating renin-angiotensin system can appropriately be called the RAAS.
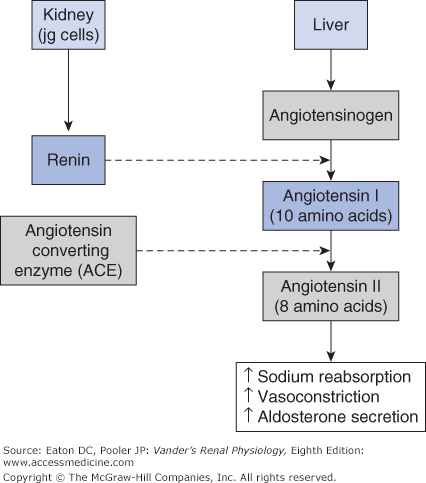
Renin-angiotensin systems are peptide-signaling systems that regulate multiple processes in the kidney and elsewhere. They consist of a protein substrate (angiotensinogen), the enzyme renin that splits off a 10 amino acid peptide (angiotensin I) from angiotensinogen, several additional enzymes that split angiotensin I into smaller peptides, and finally receptors for the peptides that activate cellular actions upon binding. The most important of these smaller peptides is the 8 amino acid peptide angiotensin II (AII). It is formed from angiotensin I by the action of angiotensin converting enzyme (ACE). AII is a mediator of multiple effects in the kidneys and elsewhere.
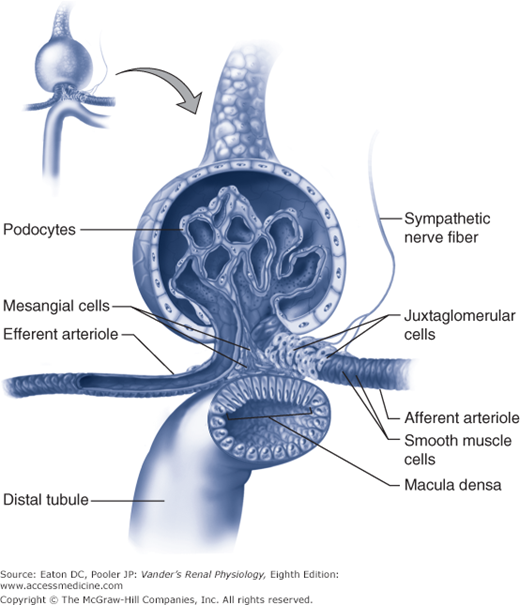
In the circulating RAAS, angiotensinogen is synthesized in the liver (Figure 7–3). Plasma angiotensinogen levels are normally high and do not limit the production of AII. Furthermore, ACE, which is expressed on the endothelial surfaces of the vascular system, particularly the pulmonary vessels, avidly converts most of the angiotensin I into AII. Therefore, the major determinant of circulating AII is the amount of renin available to form angiotensin I. As outlined in Chapter 1 and shown in Figure 7–4, renin is produced by the juxtaglomerular (jg) apparatus. The renin-secreting cells are located in the late afferent arteriole just before the glomerulus, and are referred to as juxtaglomerular granular cells (because renin can be visualized as secretory granules). The secretion of renin by the granular cells is under the control of 3 primary regulators described below.
Figure 7–3.
Major components of the systemic RAAS. Renin secreted by renal granular cells acts on angiotensinogen from the liver to produce angiotensin I. Most of the Angiotensin I is converted to angiotensin II (AII) by angiotensin converting enzyme (ACE). AII acts on the vascular system as a vasoconstrictor and stimulates adrenal production of aldosterone. AII acts within the kidneys to promote sodium reabsorption..