Rectal and vaginal drug delivery
Sanjay Garg and Josef J. Tukker
Chapter contents
Anatomy and physiology of the rectum
Absorption of drugs from the rectum
Anatomy and physiology of the vagina
Absorption of drugs from the vagina
Manufacture of rectal and vaginal dosage forms
Quality control of vaginal and rectal dosage forms
Assessment of drug release from suppositories and pessaries
Key points
• Both routes have a use in systemic drug delivery when the oral route is not available.
• These routes are relatively less popular because of their anogenital nature and privacy aspects.
Introduction
Although the oral route is the most commonly used route for drug administration, alternatives, such as parenteral, nasal, ophthalmic, rectal and vaginal may be used because they offer specific advantages. Arguments for choosing the rectal or vaginal route for drug administration include the following:
Besides these apparent advantages, the rectal and vaginal routes also have several drawbacks. These routes are generally disliked by patients because of their anogenital and sexual associations, and hence alternative routes are preferred. Depending on culture and tradition, there are strong feelings of aversion in certain countries, such as the UK and the USA, to rectal administration of drugs, whereas there is complete acceptance in continental and eastern Europe. More rational points include the slow and sometimes incomplete drug absorption and the considerable inter- and intra-subject variations. Moreover, the development of proctitis has been reported with long-term rectal delivery. There are also problems with the large-scale production of suppositories, and of achievement of a suitable shelf-life.
Thus, the rectum and vagina are not routes of first choice. Consequently, these routes of drug delivery are amongst the less desirable, resulting in the market size of the rectal and vaginal formulations being less than 1% of the total pharmaceuticals’ market.
Rectal drug delivery
Introduction
The rectal route is used in many different therapies, intended either for local or for systemic effect.
Local action.
This is desired for the local treatment of pain and itching, mostly due to the occurrence of haemorrhoids (painful, swollen veins in the lower part of the rectum and anus). Locally active drugs include astringents, antiseptics, local anaesthetics, vasoconstrictors, anti-inflammatory compounds and soothing and protective agents. Some laxatives also fall into this category.
Systemic action.
All drugs which are orally administered can be given by this route, and many are in spite of the limitations discussed above. Anti-asthmatic, anti-inflammatory and analgesic drugs are widely administered by the rectal route. Rectal preparations may also be used for diagnostic purposes.
Anatomy and physiology of the rectum
Rectal dosage forms are introduced into the body through the anus and are thus brought into contact with the most caudal part of the gastrointestinal tract, i.e. the rectum. Anatomically, the rectum is part of the colon, forming the final 150–200 mm of the gastrointestinal tract.
The rectum can be subdivided into the anal canal and the ampulla, the latter forming approximately 80% of the organ. It is separated from the outside world by a circular muscle, the anus. The rectum can be considered as a hollow organ with a relatively flat wall surface, without villi and with only three major folds – the rectal valves. The rectal wall is composed of epithelium, which is one cell layer thick, and contains cylindrical cells and goblet cells that secrete mucus. A part of the rectal wall and the rectum’s venous drainage are shown in Figure 42.1.
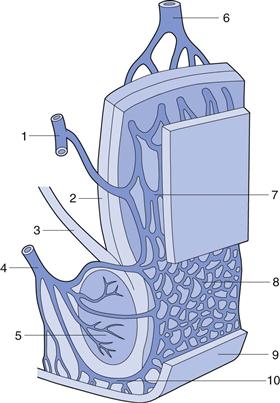
Fig. 42.1 Venous drainage of the human rectum: 1 middle haemorrhoidal (middle rectal) vein; 2 tunica muscularis; stratum longitudinale; 3 muscularis levator ani; 4 inferior haemorrhoidal (lower rectal) vein; 5 muscularis sphincter ani externus; 6 superior haemorrhoidal (upper rectal) vein; 7 and 8 plexus venosus rectalis (submucosus); 9 skin; 10 venosus marginalis. (Adapted from Tondury, 1981, with permission.)
The total volume of mucus is estimated at approximately 3 mL, spread over a total surface area of approximately 300 cm2. The pH of the mucous layer is reported as approximately 7.5 (i.e. close to neutral) in adults, and slightly alkaline in most children. Furthermore, there seems to be little buffering capacity. This point is discussed in relation to drug absorption later in this chapter.
Under normal circumstances the rectum is empty and filling provokes a defaecation reflex under voluntary control. Data comparing drug absorption from freshly prepared and aged (and therefore more viscous) suppositories suggest that there is sufficient motility to provoke spreading even of relatively viscous suppositories.
Absorption of drugs from the rectum
Absorption of drug from the rectum is primarily by passive diffusion. Because of inter-individual variations and the venous drainage of the rectum, the bioavailability of drugs following rectal administration is very unpredictable. In general, the rate and extent of drug absorption is lower than the oral route, mainly due to the small surface area for absorption.
Knowledge of the venous drainage from the rectum is important for the understanding of drug absorption. As can be seen from Figure 42.1, there are three separate veins. The lower and middle rectal (inferior and middle haemorrhoidal) veins drain into the interior vena cava, hence this blood goes directly to the heart and into the general circulation. In contrast, the upper rectal (superior haemorrhoidal) vein drains into the portal vein and therefore this blood passes through the liver before reaching the heart.
This means that drug molecules from the rectum can enter the general circulation either directly or by passing through the highly metabolizing liver. Drug absorbed in the middle and lower part of the rectum will pass directly to the general circulation and avoid first-pass metabolism in the liver. Bioavailability from the upper part of the rectum will be low for certain drugs, as much will be metabolized by the liver during its ‘first pass’ and only a proportion of the drug molecules (if they are of the high clearance type) will enter the general circulation intact.
Investigations have shown that avoiding the first-passage through the liver is possible by keeping the dosage form, and thus the released drug, in the lower part of the rectum. Compared to the small intestine, this situation is very favorable as most gastrointestinal veins drain into the portal vein.
Insertion of a suppository into the rectum results in a chain of events leading to the absorption of the drug. This is represented in a simplified scheme in Figure 42.2. Depending on the character of its vehicle (see later in this chapter), a suppository will either dissolve in the rectal fluid or melt on the mucous layer. Since the volume of rectal fluid is so small, dissolution of the complete vehicle will be difficult and requires extra water. Due to osmotic effects (of the dissolving vehicle) water is attracted, with a resultant unpleasant sensation for the patient. Independent of the vehicle type, drugs dissolved in the suppository will diffuse out towards the rectal membranes. Suspended drugs will first need to leave the vehicle (if it is water immiscible) under the influence of either gravity or motility movements and then begin to dissolve in the rectal fluid.
Dissolved drug molecules will have to diffuse through the mucous layer and then into and through the epithelium forming the rectal wall. The process of absorption will be by passive diffusion, as it is throughout the whole gastrointestinal tract for almost all drugs. Active transport processes, as found in the upper regions of the gastrointestinal tract, have not been shown to be present in the rectal area.
For a generalized discussion on drug absorption, the reader is referred to Part 4 of this book. However, some specific points concerning rectal absorption will be discussed here. Box 42.1 provides a summary of the physiological factors that are important in rectal absorption.
The quantity of fluid available for drug dissolution is very small (approximately 3 mL) spread in a layer of approximately 100 µm thick over the organ. Only under non-physiological circumstances is this volume enlarged, e.g. by osmotic attraction by water-soluble vehicles or during diarrhoea. Thus, the dissolution of poorly water-soluble drugs can easily be the slowest step in the absorptive process.
Properties of the rectal fluid, such as composition, viscosity and surface tension, are essentially unknown and have to be estimated from data available for other parts of the gastrointestinal tract. The pH and the buffering capacity of the rectum have been considered earlier in this chapter. The rectum is usually empty, except temporarily when faecal matter arrives from the colon. This material is either expelled or transported back into the colon, depending on voluntary control of the anal sphincter.
The rectal wall may exert pressure on a suppository present in the lumen by two distinct mechanisms. First, the abdominal organs may simply press on to the rectum, especially when the body is in an upright position. This may stimulate spreading and thus promote absorption. The second source of pressure is the motility of the muscles of the rectal wall, which may originate from the normally occurring colonic motor complexes. These are waves of contractions running over the wall of the colon in a caudal direction and are associated with the presence of food residues in the colon.
In contrast with the upper part of the gastrointestinal tract, no esterase or peptidase activity is present in the rectum, resulting in a much greater stability of peptide-like drugs (thus allowing attempts at their delivery by this route). Administration of these compounds using the rectal or vaginal routes has been satisfactory but only if absorption enhancers, such as surfactants are used concomitantly. All types of surfactants seem to be effective, but polyoxyethylene lauryl alcohol ether seems to have the greatest effect. One major drawback of these enhancers, however, is their irritation of the rectal mucosa in the long term. Less irritating enhancers are thus needed to explore this interesting area of drug delivery in greater depth.
Rectal dosage forms
The advantages and limitations of rectal drug administration are outlined in Box 42.2. Several categories of rectal preparations for drug delivery are available: suppositories, rectal capsules, rectal solutions, emulsions and suspensions, powders and tablets for rectal solutions and suspensions, semi-solid rectal preparations, rectal foams and rectal tampons. Of these, suppositories are the most commonly used and these are discussed first, followed by a briefer description of some of the other rectal dosage forms.
Formulation of suppositories
Suppositories are the primary, but not exclusive, dosage form used for the administration of drugs via the rectal route. Suppositories are single-dose preparations with a shape, volume and consistency appropriate for rectal administration. Administration of other suppository-type products (bougies) through other body orifices, e.g. ear, nose and urethra, is rarely undertaken and is not discussed here. Alternative dosage forms for the rectal route are discussed later.
Rectal suppositories are formulated in different shapes and sizes (usually 1–4 g). They contain one or more active substances dispersed or dissolved in a suitable base that may be soluble or dispersible in water or may melt at body temperature. Excipients such as diluents, adsorbents, surface-active agents, lubricants, antimicrobial preservatives and colouring matter may be added if necessary. Their drug content varies widely from less than 0.1% up to almost 40%.
Vehicle (suppository base)
An ideal suppository vehicle or base should melt, dissolve or disperse at body temperature. It should be non-irritating, physically and chemically stable, and pharmacologically inert. Compatibility with a range of drugs is a desirable feature. It should also be convenient to handle during manufacturing and storage. Leakage following administration is likely to be less problematic if the viscosity of the vehicle after melting or dispersion is high.
There are two main classes of vehicles in use: glyceride-type fatty bases and water-soluble bases. Although the ideal vehicle has not been found, the large variety of bases which are available enables a well-considered choice for every drug that has to be formulated into a suppository.
Choosing the optimum base requires much practical experience and can at present only partly be guided by scientifically sound data. However, some general guidelines can be given.
Requirements of the vehicle. There is no doubt that a suppository should either melt after insertion in the body or dissolve in (and mix with) the available volume of rectal fluid. For fatty bases, this means a melting range lower than approximately 37 °C (formulators should be aware that the body temperature might be as low as 36 °C at night). The melting range should be small enough to give rapid solidification after preparation, thus preventing agglomeration or sedimentation of suspended, especially high-density, drug particles. When the solidification rate is high, for example when rapid cooling is applied, this may result in fissures in the suppository. The melting temperature range, on the other hand, should be sufficiently wide to permit easy preparation, which may take a considerable length of time on an industrial scale. During solidification, a suppository should exhibit enough volume contraction to permit removal from the mould or plastic former.
The viscosity of the molten base plays an important role from both a technological and a biopharmaceutical viewpoint. During preparation, the viscosity determines not only the flow into the moulds but also the separation of drug particles. Clearly a compromise has to be found. During and after melting in the rectal cavity, the suppository mass is forced to spread over the absorbing surface. The rate of spreading is determined partly by the viscosity of the suppository at body temperature. The rate of transportation of drug particles from within the base to the interface with rectal fluid, in order to be released and absorbed, will also be affected by the viscosity.
A good suppository base should be chemically and physically stable during storage as a bulk product and after preparation into a suppository. It should have no incompatibility with, and should permit optimal release of, the drug it contains.
Clearly, this list of requirements cannot always be completely fulfilled and often an acceptable compromise is the best that can be expected.
Fatty vehicles.
The fatty vehicles in use nowadays are almost exclusively semi- or fully synthetic. Theobroma oil (also known as cocoa butter) – once a very commonly used base – is no longer used because of its many disadvantages in practice, such as its polymorphic behaviour, insufficient contraction during cooling, low softening point, chemical instability, poor water-absorptive power and its price.
A number of substitutes have been developed, which are becoming increasingly popular. The newer semi-synthetic fatty vehicles have few or none of the problems mentioned above. Commercial examples include: Cotmar, Dehydag, Fattibase, Suppocire and Witepsol. These are mixtures of natural or synthetic vegetable oils, consisting of mixed triglycerides of C12-C18 saturated fatty acids, waxes and fatty alcohols. By using a combination of components, they can be designed to have an adjustable range of melting points, e.g. different grades of Witepsol have melting points ranging from 29 to 44 °C.
The ‘hydroxyl number’ of these bases is a parameter that refers directly to the amount of mono- and di-glycerides present in the fatty base. A high number means that the base is less hydrophobic and its power to absorb water is high. This may lead to an increased rate of decomposition for drugs that are easily hydrolysed. This capacity could also lead to the formation of a w/o emulsion in the rectum. This is generally to be avoided because of a very low drug release rate. An advantage of a high hydroxyl number is the larger melting and solidifying ranges that permit easier manufacture.
Water-soluble vehicles.
Hydrophilic water-soluble (or miscible) vehicles are much less frequently used. They comprise the classic glycerinated gelatin (glycerol-gelatin) and polyethylene glycol (macrogol) bases. Glycerol-gelatin bases are mostly used for laxative purposes and in vaginal dosage forms (see below).
Glycerinated gelatin is a mixture of glycerol, gelatin and water. The mixture forms a translucent, gelatinous mixture, dispersible in the rectum. The ratio of glycerol, gelatin and water can affect the dispersion time and thus the duration of action. A higher proportion of gelatin in the mixture makes it more rigid and longer acting. The following is an example composition of the base:
Preservatives such as methyl and/or propyl parabens may be added. Formulations made with gelatin and glycerol tend to be hygroscopic and require well-closed containers for packaging.
Polyethylene glycols (PEGs) are very versatile polymers in their properties and applications. They consist of mixtures of polyethylene glycols of different molecular weight. Lower molecular weight PEGs (PEG 400 and 600) are liquid, those around 1000 are semi-solid and those above 4000 are waxy solids. Different molecular weights of PEG can be combined to produce the desired properties, as seen in Table 42.1.
Table 42.1
Compositions of PEG bases with different physical characteristics
| Base A | Base B | |
| PEG 1000 | 95% | 75% |
| PEG 4000 | 5% | 25% |
| Properties | Low melting temperature, immediate drug release | Higher melting temperature, sustained drug release |
The melting point of these vehicles is well above body temperature, which means that they need to mix with the rectal fluid. PEGs of all molecular weights are miscible with water and rectal fluids, thereby releasing drug by dispersion; the available volume of rectal fluid is too small for true dissolution. Because of their high melting point, PEG-based formulations are especially suited for application in tropical climates but several disadvantages have to be considered.
They are hygroscopic and therefore attract water after administration, resulting in an uncomfortable sensation for the patient. Incorporation of at least 20% water in the base and moistening before insertion can help to reduce this problem. A considerable number of incompatibilities with various drugs have been reported. Due to the solubilizing character of this base (which has a low dielectric constant) drugs may tend to remain in the base, and drug release may be slow.
PEG bases can develop peroxides on storage, therefore airtight packaging is recommended and the formulation should be monitored for peroxides during stability studies when ascertaining shelf-life.
Choice of vehicle.
A summary of the points which are important for the choice of a suppository base is given in Box 42.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree