TERMS
•Partial parenteral nutrition
•Reconstitution
•Solute
•Solution
•Suspension
•Syrup
•Total parenteral nutrition
OBJECTIVES
Upon completion of this chapter, the technician student will be able to:
•Define diluent, solute, suspension, and solution.
•Determine the amount of solvent required to prepare oral and parenteral products in a desired strength.
•Properly document reconstituted products for storage and later usage.
This chapter discusses the reconstitution of various medications, including oral powders and powders for parenteral administration. The student technician not only is introduced to the standard directions but also learns to modify the reconstitution directions to accommodate the patient’s needs, or in other words, change the concentration of prepared powders for solution.
 Constitution of Powders
Constitution of Powders
Oral Solution or Oral Suspension
Some drugs, most notably antibiotics, lose their potency in a relatively short time when prepared in a liquid dosage form. Thus, to enhance the shelf life of these drugs, manufacturers provide products to the pharmacy in powder form for reconstitution with purified water or special diluent at the time a prescription or medication order is received. Depending on the product, the powder may be stable for about 24 months. After reconstitution, the solution or suspension is stable in the quantities usually dispensed for up to 10 days at room temperature or 14 days under refrigeration.
Powders (solute) for reconstitution are packaged in self-contained bottles of sufficient size to accommodate the addition of the required volume of diluent (Fig. 12.1). In addition to the therapeutic agent, the powder contains such ingredients as solubilizing or suspending agents, stabilizers, colorants, sweeteners, and flavorants.
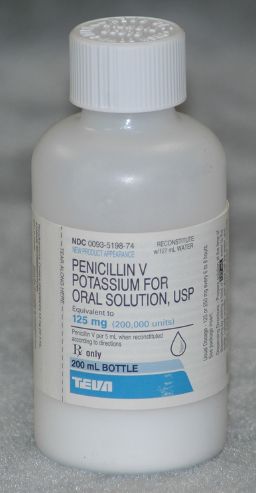
Figure 12.1Penicillin VK 125 mg/5 mL suspension is one type of powder packaged for reconstitution.
solute The substance being dissolved or diluted; most often solid but sometimes liquid.
On receipt of a prescription order, the pharmacist or pharmacy technician follows the label instructions for reconstitution ), adding the proper amount of purified water or other diluent to prepare the liquid form. Some labeling instructions use the term constitute rather than reconstitute in describing the general process. If a powder is prepared from its original solution by removing the solvent (as by freeze-drying) and then the solution is restored by the pharmacist, the term reconstituted is correct. Some injectable products are prepared in this fashion.
reconstitution Adding a diluent, such as purified water or other liquid, per package directions, to a powder to make a solution or suspension.
Depending on the product’s formulation, reconstitution results in the preparation of a suspension (Fig. 12.2) or a clear solution (often called a syrup). The final volume of product is the sum of the volume of solvent or diluent and the volume of the dissolved or suspended powder mixture. These products generally are intended for infants and children but also may be used by adults who have difficulty swallowing tablets and capsules.

Figure 12.2 (A) A pharmacy technician reconstituting an oral powder for suspension. (B) On the left is a bottle of cephalexin powder for oral suspension; on the right is a reconstituted bottle of cephalexin ready to be dispensed.
suspension Preparation containing finely divided drug particles dispersed in a liquid vehicle.
solution Liquid preparation that contains one or more solutes in a solvent or mixture of solvents.
syrup Concentrated aqueous solution of a sugar or sugar substitute; may or may not be medicated.
For children and adults, reconstituted products for oral solution or suspension generally are formulated such that the usual dose of the drug is contained in teaspoonful amounts of the product. For infants, pediatric drops, which also require reconstitution, may be used. Sometimes these products must be mixed, changing their normal concentration, to make dosing easier for the patient or caregiver.
Rules for Changing Concentration of Products

1. The amount of drug in the package does not change.
2.The powder volume in the package does not change.
3.It is possible to change the concentration of the drug by adding more or less diluent.
The calculations that follow demonstrate the preparation of reconstituted products and how the drug concentrations may be designed to meet a particular patient’s requirements.
Concentration After Reconstitution
Calculating the concentration of an oral liquid after reconstitution according to the manufacturer’s directions entails the following.
Example:
The label for a powder package of penicillin V potassium for oral solution directs the pharmacist to dissolve the powder in the container with sufficient purified water to make 200 mL of solution. If the package contains 5 g of penicillin V potassium, how much (in milligrams) of the antibiotic is contained in each 1-tsp dose of the resultant solution?
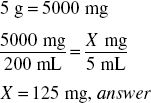
Solve by Dimensional Analysis
![]()
Volume of Powder and Total Drug Content
Calculating the volume of powder and total drug content of a product to be reconstituted for oral solution or suspension entails the following.
Example:
Label instructions for an ampicillin product call for the addition of 78 mL of water to make 100 mL of reconstituted suspension such that each 5 mL contains 125 mg of ampicillin.
Volume of powder:
Because the addition of 78 mL of water results in preparation of 100 mL of product, the volume occupied by the powder is
100 mL − 78 mL = 22 mL, answer
Total drug present:
If in the reconstituted product, each 5 mL contains 125 mg of ampicillin, the total amount of ampicillin in the 100-mL product is
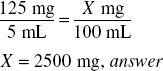
Modifying Label Instructions to Obtain Desired Concentration
Remember, the amount of solute (powdered drug) does not change with the addition of the diluent (solvent). By the addition of more or less diluent (solvent) it is possible to change the strength of the product. Chapter 10 notes that if the amount of active ingredient remains constant, any change in the quantity of a solution or mixture of solids is inversely proportional to the percentage or ratio strength; that is, the percentage or ratio strength decreases as the quantity increases, and conversely. In other words, if the amount of active ingredient remains the same and the volume gets larger, the concentration gets lower. Because of this principle we can modify the label instructions.
When the volume of diluent is decreased, there must still be enough for the solute to dissolve completely.
To modify the label instructions to prepare a reconstituted product of a desired concentration, observe the following.
Example:
Using the product in the previous example, if a physician desires an ampicillin concentration of 100 mg in 5 mL rather than 125 mg in 5 mL, how much water (in milliliters) should be added to the powder?
The dry product contains 2500 mg of ampicillin. The volume of product that can be made with a concentration of 100 mg/5 mL may be calculated by:
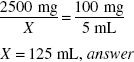
The powder occupies 22 mL of volume. To determine the amount of water to add:
125 mL − 22 mL = 103 mL, answer
Reconstitution Based on Dosage Calculation of Weight and Body Surface Area
To reconstitute a powder into an oral solution or suspension based on dosage calculation of weight or body surface area (BSA), observe the following.
Examples:
The label of a powder for oral suspension states that when 111 mL of water is added, 150 mL of a suspension containing 250 mg of ampicillin in 5 mL is prepared. How much purified water (in milliliters) should be used to prepare, in each 5 mL of product, the correct dose of ampicillin for a 60-lb child who is prescribed 8 mg/kg of body weight?
The dose of ampicillin may be determined by
![]()
The amount of ampicillin in the container is determined by:
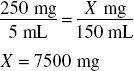
Thus, the amount of product that can be made from 7500 mg of drug such that each 5 mL contains 218 mg of drug may be found by:
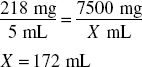
Finally, because the volume of powder occupies 39 mL (150 mL − 111 mL), the amount of water to add is determined by:
172 mL − 39 mL = 133 mL, answer
The label of a powder for reconstitution into pediatric drops states that when 12 mL of purified water is added to the powder, 15 mL of a pediatric suspension containing 50 mg of amoxicillin per milliliter results. The medication dropper delivers 20 drops/mL, the child has a BSA of 0.4 m2, and the dose of the drug is based on 50 mg/m2 of BSA. How much water (in milliliters) should be added to prepare the dose of amoxicillin in each 10 drops?

Subtracting the volume accounted for by the powder (15 mL − 12 mL = 3 mL), the volume of water to add is determined by
18.75 mL − 3 mL = 15.75 mL, answer
Parenteral Use
Because of instability in liquid form, some medications (particularly antibiotics) intended for injection are provided as powder in vials to be reconstituted with sterile water or other designated solvent or diluent for injection immediately before use. Generally these medications are small-volume products intended for use by injection or as additives to large-volume parenterals.
In contrast to the powders intended for oral use after reconstitution, injectable products may contain only limited amounts of specified added ingredients to increase the stability and effectiveness of the drug (obviously, no colorants, flavorants, sweeteners, and so on are needed). In effect, the bulk volume of the dry content of a vial is largely or entirely the medication.
If the quantity of the dry drug powder is small and does not contribute significantly to the final volume of the reconstituted solution, the volume of solvent used approximates the final volume of solution. For example, if 1000 units of a certain antibiotic in dry form is to be dissolved and if the powder does not account for any significant portion of the final volume, the addition of 5 mL of solvent will produce a solution containing 200 units/mL. However, if the bulk of the powder contributes to the final volume of the reconstituted solution, the increase in volume produced by the drug must be considered, and this factor must be used in calculating the amount of solvent needed to prepare a solution of a desired concentration. For example, the package directions for making injectable solutions of piperacillin sodium specify that 4 mL of sterile solvent should be added to 2 g of the powder to produce 5 mL of a solution that is to contain 400 mg/mL. The drug, in this case, accounts for 1 mL of the final volume. Again, adding 20 million units of penicillin G potassium (Fig. 12.3) to 32 mL of sterile solvent provides a total volume of 40 mL of a solution that contains 500,000 units/mL. The powder now accounts for 8 mL of the final volume.
Figure 12.3This vial of penicillin G potassium has 20 million units.
Solvent Needed to Produce Desired Concentration When Dry Agent Does Not Contribute to Final Volume
Calculating the amount of solvent needed for a given amount of powder to produce a desired concentration when the powder does not account for any significant portion of the solution entails the following.
Examples:
Using a vial containing 200,000 units of penicillin G potassium, how much solvent (in milliliters) should be added to the powder to prepare a solution having a concentration of 25,000 units/mL?
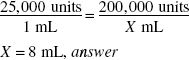
Assume a vial contains 200,000 units of penicillin G sodium and the solvent is sodium chloride injection. Explain how you would obtain the penicillin G sodium needed for the following prescription.

15,000 units × 10 = 150,000 units of penicillin G sodium needed.
Because the powder is 200,000 units of penicillin G sodium, or ![]() times the number of units desired, ¾ of the powder will contain the required number of units.
times the number of units desired, ¾ of the powder will contain the required number of units.
Step 1. Dissolve the powder in 4 mL of sodium chloride injection.
Step 2. Use 3 mL of the reconstituted solution, answer
Solve by Dimensional Analysis
![]()



