Rare and Unusual Disorders of the Female Genital Tract
This chapter contains descriptions of uncommon benign and malignant lesions, that for the most part, may affect several component organs of the female genital tract.
RARE BENIGN DISORDERS
Deficiency of Folic Acid
Deficiency in folic acid (or vitamin B12) leads to impaired DNA synthesis during hematopoiesis, resulting in abnormal maturation of erythrocytes (and other blood cells) known as megaloblastic anemia. The disease is characterized mainly by a marked enlargement of erythrocytes and abnormalities of leukocytes. Folic acid deficiency may also impair DNA synthesis in other organs, such as the oral cavity and the gastrointestinal tract, where it can cause cellular enlargement (see Chaps. 21 and 24). In 1962 and 1966, Van Niekerk reported cell changes observed in squamous cells in cervical smears in patients with megaloblastic anemia and, hence, a folic acid deficiency, during the puerperium. The principal changes observed were generalized enlargements of intermediate squamous cells (diameter of 70 μm or larger), accompanied by an enlargement of the nucleus (diameter of 14 μm or more). Van Niekerk also observed multinucleation and cytoplasmic vacuolization in an average of 3.5% of cells. Other findings included phagocytosis, clumping, and folding of nuclear chromatin. The changes were apparently reversible after appropriate therapy. Van Niekerk’s observations were generally confirmed by Klaus (1971), who considered nuclear folding as the most frequent event (6% of cells) and nuclear enlargement the second most frequent event (4% of cells). In Klaus’ experience, the cytologic changes may be observed 8 to 10 weeks before clinical onset of megaloblastic anemia. Subsequently, Whitehead et al (1973) linked similar cell changes
with contraceptive therapy but the conclusive proof of this association is lacking.
with contraceptive therapy but the conclusive proof of this association is lacking.
Changes apparently caused by folic acid deficiency are deceptively similar to early neoplastic changes in the uterine cervix such as the dyskaryosis (dysplasia) of the superficial and intermediate squamous cells, consistent with a low-grade squamous intraepithelial lesion, and abnormalities caused by a human papillomavirus infection (see Chap. 11). It is of interest in this regard that folic acid deficiency may apparently enhance the patient’s susceptibility to infection with human papillomavirus (Harper et al, 1994; Butterworth et al, 1992). Somewhat similar cell abnormalities may be observed as an early effect of radiotherapy (see Chap. 18). Because the cytologic follow-up of such lesions may be unreliable, it is prudent to have the patient undergo an appropriate work-up that should include a colposcopic evaluation of the uterine cervix before accepting the diagnosis of folic acid deficiency as secure.
Pemphigus Vulgaris
Pemphigus vulgaris (from Greek, pemphis = blister) is a disorder usually affecting the skin and the mucous membranes of the oral cavity (see Chapter 21). It may sometimes involve the lower female genital tract. Several such cases were described in the gynecologic and dermatologic literature (see Krain et al, 1973). The disease is caused by antibodies to desmoglein 3, a component protein of desmosomes (Amagai et al, 1996) causing a disruption of desmosomes in the lower layers of the squamous epithelium leading to the formation of fluid-filled blisters, vesicles or bullae. The latter contain atypical squamous cells (cells of Tznack et al, 1951, who first described them) that can be observed in smears of broken vesicles. These are squamous cells of bizarre shapes, sometimes with cytoplasmic protrusions, characterized by clear cytoplasm and nuclei and large nucleoli, occurring singly and in clusters. The antibodies coating these cells can be demonstrated by immunofluorescence, as first shown by Beutner and Jordan in 1964. Libcke (1970) and Friedman et al (1971) each reported atypical squamous cells in cases of pemphigus vulgaris involving the squamous epithelium of the cervix. A number of additional cases of genital pemphigus, some also involving the vulva and vagina, were described (Kaufman et al, 1969). In a case described by Valente et al (1984), the atypical squamous cells were interpreted as suspicious, yet corresponded to clinically occult pemphigus blisters discovered in the hysterectomy specimen. In a case reported by Dvoretsky et al (1985), pemphigus was associated with a microinvasive carcinoma of the uterine cervix and, in a case reported by Krain et al (1973), with endometrial carcinoma. Because genital pemphigus can cause vaginal bleeding, it is obvious that a thorough examination of the female genital tract is required to rule out the possibility of a malignant tumor associated with this disease. For further discussion and illustrations of pemphigus, see Chapters 21 and 34.
Malakoplakia
This rare disorder of macrophages, unable to cope with colibacteria because of an enzymatic deficiency, is described in detail and illustrated in Chapter 22. The characteristic, spherical cytoplasmic Michaelis-Guttmann bodies (representing enlarged and often calcified lysosomes), observed in medium size macrophages, are diagnostic of this disorder in smears. The findings in cervicovaginal smears were described in several cases of malakoplakia involving the vagina and uterine cervix (Lin et al, 1979; Chalvardijan et al, 1980; Wahl, 1982; Valente et al, 1984; Falcon-Escobedo et al, 1986). In an electron microscopic study, Kapila and Verma (1989) identified the characteristic coliform bacteria within the Michaelis-Guttmann bodies in a case of cervical malakoplakia. Thomas et al (1978) described a case of this disorder involving the endometrium and causing abnormal bleeding.
Amyloidosis of the Cervix
A case of amyloidosis limited to the uterine cervix was reported by Yamada et al (1988). There are no known cytologic findings in this very rare disorder.
Eosinophilic Granuloma (Langerhans’ Cell Granulomatosis)
This uncommon lesion, composed of Langerhans cells resembling macrophages and a mixture of eosinophilic polymorphonuclear leukocytes with other inflammatory cells, is known to involve the female genitals (Zinkham, 1976; Issa et al, 1980). There is no information on the cytologic presentation of this lesion in cervicovaginal smears. For discussion of this entity in other organs, see Chapters 19 and 35.
Ectopic Prostatic Tissue in the Uterine Cervix
Larraza-Hernandez et al (1997) and Nucci et al (2000) reported the presence of ectopic prostatic tissue in the uterine cervix, presenting in one case as a cervical mass (thought to be a fibroid) and in three cases as an incidental finding in tissue obtained for treatment of high-grade squamous intraepithelial lesions.
BENIGN TUMORS
Leiomyomas
These benign tumors of smooth muscle usually involve the myometrium of the body of the uterus and may reach substantial sizes. They are virtually always encapsulated and do not normally shed any cells in cytologic samples from the uterus. Occasionally, however, ulcerated leiomyomas of the uterus, particularly if located in the uterine cervix, may shed benign smooth muscle cells that can be recognized in cervicovaginal smears. These slender cells are spindly, elongated, usually occur in parallel bundles, and show oval, finely granular nuclei, often located in the approximate center of the cell. Similar cells may be sometimes observed following abortion and after a mechanical injury to the
cervix (see Chap. 8). Endometrial abnormalities may occur in the presence of large leiomyomas (see Chap. 13).
cervix (see Chap. 8). Endometrial abnormalities may occur in the presence of large leiomyomas (see Chap. 13).
Other Benign Tumors
Other benign tumors of the uterus, vulva or vagina, such as rhabdomyoma (Gad and Eusebi, 1975; Gold and Bossen, 1976), syringoma (Young et al, 1980), glomus tumor (Spitzer et al, 1985), and granular cell tumor (Coates and Hales, 1973), are exceedingly rare and they have not been observed in smears. Granular cell tumors of the breast, and sometimes of other organs, may be recognized in aspiration biopsies (see Chaps. 20 and 29).
RARE MALIGNANT TUMORS
Sarcomas
Sarcomas and other malignant tumors of mesenchymal origin constitute about 2% to 3% of all malignant tumors of the female genital tract. Their most common primary site is the uterine corpus, followed by the cervix and vagina. Most sarcomas are homologous (i.e., made up of a single tissue type, such as smooth or striated muscle or fat). However, a substantial number of malignant mesenchymal tumors of the uterus, known as the mesodermal or Müllerian mixed tumors, contain a mixture of epithelial and sarcomatous components. The histologic and cytologic aspects of these and other sarcomas are discussed below.
Most sarcomas originate within the depths of the affected organ and do not reach the exfoliating surface until they have grown to a substantial size and have produced surface ulceration. In the vast majority of such cases, the patients are symptomatic and have clinically obvious disease. Thus, routine cytologic examination of the female genital tract rarely contributes to the primary diagnosis of these tumors, except for the mesodermal mixed tumor. In most cases, the role of cytology is relegated to the recognition of recurrent disease. In many instances, even this exercise is fraught with considerable difficulty. Occasionally, however, cytologic evaluation may contribute to the diagnosis and clinical handling of the patient.
Tumors of Smooth Muscle (Leiomyosarcomas)
Histology
Leiomyosarcomas are the most common sarcomas of the female genital tract. Nearly all tumors originate in the smooth muscle of the uterine corpus, although they may be primary in the muscle of the uterine cervix and, exceedingly rarely, in other genital organs such as the fallopian tube or the wall of the vagina. The tumors are composed of crisscrossing bundles of abnormal smooth muscle cells, characterized by large, hyperchromatic nuclei. Several rare variants of these tumors are known to occur, chief among them the epithelioid leiomyosarcoma, a tumor composed of large, polygonal cells mimicking an epithelial tumor. The prognosis of uterine leiomyosarcomas depends on the size of the tumor, its relationship to adjacent organs, and its histologic differentiation or grade. Small tumors incidentally found within the myometrium or arising within benign leiomyomas generally offer an excellent prognosis. However, tumors attached to adjacent viscera are often fatal, regardless of grade. Well-differentiated tumors, closely resembling benign leiomyomas, except for nuclear abnormalities and sometimes high mitotic count (grade I), usually have a much better prognosis than tumors composed of bundles of clearly malignant cells (grade II). Highly disorganized tumors made up of bizarre large or small cancer cells (grades III and IV) have a nearly uniformly fatal prognosis (Spiro and Koss, 1965; Bodner et al, 2003). Voluminous literature pertaining to the classification and recognition of leiomyosarcomas, particularly the differentiation between atypical leiomyomas and low-grade leiomyosarcomas (Bell et al, 1994), has very limited bearing on cytologic observations.
Cytology
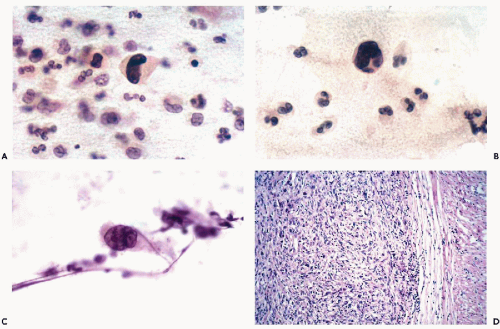
Massoni and Hajdu (1984) stressed the very low rate of primary leiomyosarcomas recognized in cervicovaginal material. Cells from the well-differentiated forms of leiomyosarcoma (grade I) have never been seen by us or identified in routine smears. However, more anaplastic forms of this tumor (grades II through IV), once ulcerated or metastatic, may shed highly abnormal, often grotesque cancer cells. If such cells are elongated, as is sometimes the case, a more specific diagnosis of tumor type may be attempted (Fig. 17-1). Single or multiple abnormal nuclei of variable sizes may be noted. Nuclear hyperchromasia is variable, and irregular, large nucleoli may be present. The difference between cells of a high grade leiomyosarcoma and normal smooth muscle cells is obvious. Quite often, however, cancer cells shed from a leiomyosarcoma are polygonal rather than elongated and may be mistaken for cells of a carcinoma.
Elongated, spindly, or bizarre malignant cells are not unique to leiomyosarcomas and may occur in other sarcomas and in mesodermal mixed tumors. Similar cells may also occur in invasive epidermoid carcinomas, particularly of the spindle- and giant-cell variety (see below).
Klijanienko et al (2003) reported a large series of leiomyosarcomas of various types and primary extrauterine locations, diagnosed by direct thin needle aspiration. To my knowledge, no attempts have been made to apply this technique to uterine tumors.
Tumors of Striated Muscle (Rhabdomyosarcomas)
Although the female genital tract does not normally contain striated muscle, isolated cells of this type may occasionally be found in benign myometria. This, however, is not an essential prerequisite for the occurrence of rhabdomyosarcoma, which apparently may originate from any type of mesenchymal cell. Pure embryonal-type sarcomas of striated muscle origin are most commonly observed in the vagina or the cervix of young children and young adults as botryoid sarcomas (Daya and Scully, 1988). Occasional rhabdomyosarcomas of alveolar type have been observed in other organs of the female genital tract, for example, in the uterine corpus (Donkers et al, 1972) and the vulva (Imachi et al, 1991).
Rhabdomyosarcomas are a common component of mesodermal mixed tumors (see below).
Rhabdomyosarcomas are a common component of mesodermal mixed tumors (see below).
Botryoid sarcoma (from the Greek, botrys = bunch of grapes) is a form of immature (embryonal) rhabdomyosarcoma that forms grape-like, translucent tumor nodules usually in the vagina, much less commonly in the uterine cervix of young girls, who are rarely older than five years of age, but sometimes also in young adults. Similar tumors may occur in the urinary bladder of children of both sexes and in the prostate of boys. The grape-like structures are surfaced by an intact squamous epithelium. The tumor cells form a dense subepithelial layer (cambium layer) composed of very small cancer cells, surrounding the loosely structured bulk of the tumor wherein larger cancer cells, some with cytoplasmic cross-striations or marked cytoplasmic eosinophilia, may be identified.
Previously considered nearly invariably fatal (Daniel et al, 1959), the tumors are now curable in a large proportion of cases with a combination of radiotherapy and chemotherapy (review in Brand et al, 1987). Daya and Scully (1988) stressed better prognosis of botryoid sarcoma of the uterine cervix in young adults than in children.
Cytology
The diagnosis of primary botryoid sarcomas is usually made by clinical inspection and biopsy. Cytologic diagnosis is superfluous in such instances. Even if vaginal smears are obtained, the tumor cells may be absent because of the protective epithelial layer. However, in recurrent and in metastatic tumors, small, elongated, spindly tumor cells may be observed in vaginal smears or in urinary sediment (see Chap. 26). In rare instances, cytoplasmic cross-striations may occur that allow a precise classification of the tumor.
Differentiated rhabdomyosarcomas are very rare in the female genital tract (Brand et al, 1989). One can then anticipate the finding of bizarre tumor cells with cytoplasmic cross-striations, characteristic of rhabdomyoblasts (see Fig. 17-6). The cytologic findings in two cases of alveolar rhabdomyosarcoma of the vulva were reported by Imachi et al (1991), who did not observe cytoplasmic striations in the tumor cells.
Endometrial Stromal Sarcomas
The endometrial stromal tumors originate either in the endometrium or in foci of uterine endometriosis (adenomyosis). There are two presentations of this tumor: a low-grade and a high-grade tumor.
Histology and Clinical Features
The low-grade, well-differentiated form of the tumor (previously named the endolymphatic stromal myosis) is composed
of orderly bundles of small cells similar to endometrial stroma, sometimes forming ribbon-like organoid structures (so called “plexiform tumorlets”) and occasionally small glands, mimicking primitive endometrial glands. Clement and Scully (1989) misinterpreted the “tumorlets” for sex cord-like elements, seen in rare ovarian tumors, but the origin of these structures from endometrial stroma has been clearly documented by Larbig et al (1965). The tumor cells may occasionally differentiate into smooth muscle cells, particularly in metastatic foci. Oliva et al (2001) pointed out that cells with markedly eosinophilic cytoplasm may occur in such tumors. The tumor has several other interesting features: it has the tendency to invade vessels of the uterus and adjacent pelvis, sometimes forming solid, spaghetti-like cylinders that can be pulled from the affected vessels by a forceps. For this reason, the tumor may be confused with intravenous leiomyomatosis, as in a paper by Clement et al (1988). The tumor may have an erratic, protracted clinical course, stretching over a period of many years (Koss et al, 1965). It may form local, retroperitoneal, or distant metastases, for example, to the bladder or lung, often many years after surgical removal of the primary tumor (28 years in a personally observed case), and still be consistent with long-term survival following aggressive treatment of metastases. Late pulmonary metastases were also described by Abrams et al (1989). The tumor may also respond to hormonal manipulation with progesterone, not unlike an endometrial carcinoma. Because of the unusual, often favorable behavior of this tumor, its diagnosis in metastatic foci may be life-saving.
of orderly bundles of small cells similar to endometrial stroma, sometimes forming ribbon-like organoid structures (so called “plexiform tumorlets”) and occasionally small glands, mimicking primitive endometrial glands. Clement and Scully (1989) misinterpreted the “tumorlets” for sex cord-like elements, seen in rare ovarian tumors, but the origin of these structures from endometrial stroma has been clearly documented by Larbig et al (1965). The tumor cells may occasionally differentiate into smooth muscle cells, particularly in metastatic foci. Oliva et al (2001) pointed out that cells with markedly eosinophilic cytoplasm may occur in such tumors. The tumor has several other interesting features: it has the tendency to invade vessels of the uterus and adjacent pelvis, sometimes forming solid, spaghetti-like cylinders that can be pulled from the affected vessels by a forceps. For this reason, the tumor may be confused with intravenous leiomyomatosis, as in a paper by Clement et al (1988). The tumor may have an erratic, protracted clinical course, stretching over a period of many years (Koss et al, 1965). It may form local, retroperitoneal, or distant metastases, for example, to the bladder or lung, often many years after surgical removal of the primary tumor (28 years in a personally observed case), and still be consistent with long-term survival following aggressive treatment of metastases. Late pulmonary metastases were also described by Abrams et al (1989). The tumor may also respond to hormonal manipulation with progesterone, not unlike an endometrial carcinoma. Because of the unusual, often favorable behavior of this tumor, its diagnosis in metastatic foci may be life-saving.
The high grade endometrial stromal sarcomas are infrequent. In a small series by Koss et al (1965), only 1 of 10 stromal sarcomas could be so classified. The tumor has a similar distribution to the low-grade variant but is composed of obviously malignant larger cells and has aggressive behavior.
Cytology
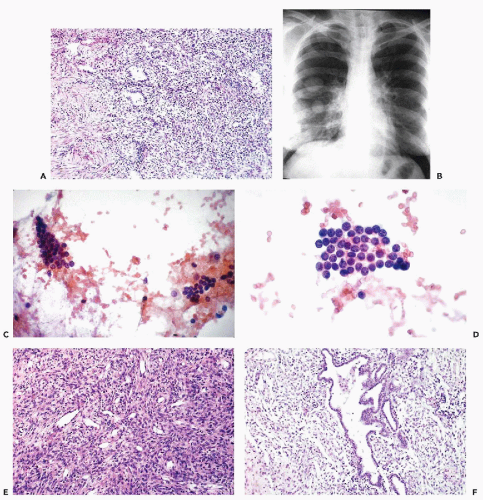
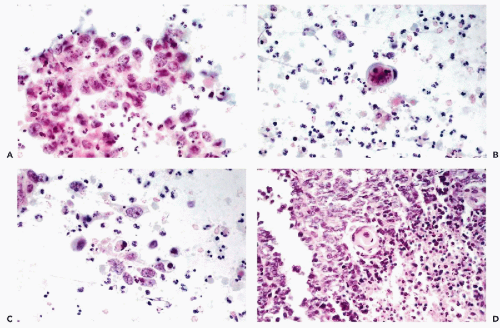
Several examples of this tumor were described in cervicovaginal smears (Hsiu and Stawicki, 1979; Becker and Wong, 1981) and in other cytologic samples such as effusions (Massoni and Hajdu, 1984; Hajdu and Hajdu, 1976). So far as one can tell, the reported cases represented the high-grade variant of the disease. In general, the authors stressed the small size and the relatively monotonous appearance of the round or oval malignant cells, accompanied by occasional elongated cells with tapering cytoplasm, named comet cells by Hsiu and Stawicki (1979). Except for hyperchromasia and variability in size, the nuclei had no distinguishing features. Mitotic figures were observed in two of three cases reported by Becker and Wong (1981). In all cases described, the tumor was far advanced and symptomatic; all patients, save one, died of disease shortly after diagnosis.
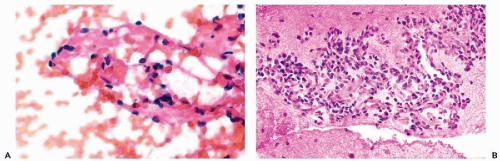
There are no reported cases of primary diagnosis of the low-grade stromal sarcoma. However, metastases of this tumor may be amenable to diagnosis by needle aspiration biopsy that may lead to aggressive treatment and long-term survival. As an example, the aspirate of one of many large, cannon-ball pulmonary metastases in a 38-year-old woman contained small, spindly, rather benign-looking cells and scattered glands, resembling benign endometrial glands (Fig. 17-2). The cytologic finding led to the review of hysterectomy material obtained 8 years previously, which was initially diagnosed as a benign abnormality (stromal nodule). The review disclosed a low-grade endometrial stromal sarcoma. After surgical removal of all but one of the pulmonary metastases, followed by progesterone therapy, the patient remained well for several years without any evidence of active disease. In another more recent case with only short follow-up, the aspirate of lung metastases yielded small cells resembling normal endometrial stroma (Fig. 17-3).
Other Sarcomas
Exceedingly uncommon sarcomas may be observed in the female genital tract.
Epithelioid sarcomas are very rare sarcomas of soft tissue that characteristically mimic epithelial tumors, hence their name. A few cases of this disease involving the vulva were reported in the cytologic literature (Ulbright et al, 1983; Hernandez-Ortiz et al, 1995). In aspirated material, the authors reported the presence of polygonal malignant cells with eosinophilic cytoplasm, large nuclei and prominent nucleoli, mimicking cells of a clear cell carcinoma. It is unlikely that an accurate cytologic diagnosis of these tumors can be established in the absence of clinical history.
A case of malignant fibrous histiocytoma of the cervix, with cytologic findings, was described by Fukuyama et al (1986). The cytologic features included multinucleated and elongated (spindly) cancer cells. Zaleski et al (1986) and Foschini et al (1989) each reported a case of alveolar soft-part sarcoma of the vagina. In keeping with the pseudoepithelial histologic appearance of this tumor, large malignant cells, with eosinophilic granular cytoplasm, singly and in clusters, were observed in the cervicovaginal smears. The nuclei were eccentric and provided with large nucleoli. Characteristic intracytoplasmic crystalloids were documented by periodic acid-Schiff (PAS)-stain and by electron microscopy.
An osteosarcoma, a liposarcoma, and a Wilms’ tumor of the uterine cervix were described (Bloch et al, 1988; Bell et al, 1985; Brooks and LiVolsi, 1987) but there is no information on their cytologic presentation in this anatomic location. A synovial sarcoma-like tumor of the vagina was described by Okagaki et al (1976). For cytologic presentation of sarcomas of various types and organs in aspiration biopsies, see Chapter 35.
Malignant Mesodermal Mixed Tumors (Müllerian Mixed Tumors)
The highly malignant mesodermal mixed tumors are most often of endometrial origin; similar tumors, however, may also occur in the uterine cervix, fallopian tube, the ovary, and organs of other than Müllerian origin, such as the urinary bladder (Mortel et al, 1974; Dictor, 1985; Wu et al, 1973; see also Chapter 23). Hence, the commonly used term “Müllerian mixed tumors,” is not accurate. In the female genital tract, the most common mesodermal mixed tumors of the endometrium occur chiefly in the menopausal age group and often appear clinically as polypoid lesions, sometimes protruding through the external os of the cervix.
When these tumors show only elements of carcinoma with spindle cell stroma, they are usually classified as carcinosarcomas or spindle cell carcinomas (see below).
Histology
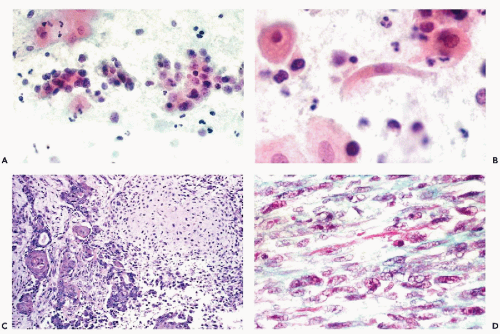
These tumors are composed of a mixture of undifferentiated and differentiated sarcomas and carcinomas. The most common differentiated sarcoma is rhabdomyosarcoma (see Fig. 17-4C,D). Other sarcomatous elements may resemble endometrial stromal sarcoma, leiomyosarcoma,
chondrosarcoma, or liposarcoma (see Fig. 17-5D). The carcinomatous components are in the form of adenocarcinoma, squamous carcinoma, or a mixture of both. Clement and Scully (1974) identified a subvariant of mesodermal mixed tumors in which the epithelial component was morphologically benign and named it adenosarcoma. However, the behavior of adenosarcoma was similar to that of malignant mesodermal mixed tumor. In 1989, the same authors described a very rare variant of mesodermal mixed tumor with sex-cord-like elements. An exceedingly rare, histologically benign variant of mesodermal mixed tumor has been described (Vellios et al, 1973; Demopoulos et al, 1973).
chondrosarcoma, or liposarcoma (see Fig. 17-5D). The carcinomatous components are in the form of adenocarcinoma, squamous carcinoma, or a mixture of both. Clement and Scully (1974) identified a subvariant of mesodermal mixed tumors in which the epithelial component was morphologically benign and named it adenosarcoma. However, the behavior of adenosarcoma was similar to that of malignant mesodermal mixed tumor. In 1989, the same authors described a very rare variant of mesodermal mixed tumor with sex-cord-like elements. An exceedingly rare, histologically benign variant of mesodermal mixed tumor has been described (Vellios et al, 1973; Demopoulos et al, 1973).
Cytology
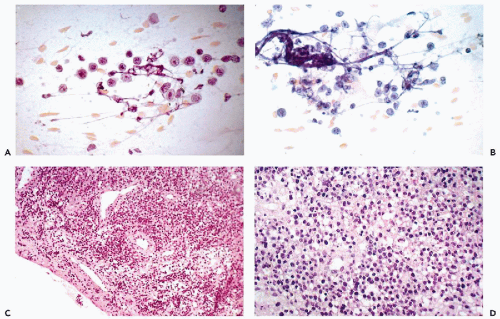
The mesodermal mixed tumors may sometimes be recognized in cervicovaginal smears prior to clinical diagnosis. The background of the smears is usually filled with necrotic material and fresh and old blood. Fully developed mesodermal mixed tumors usually shed abundant cancer cells (Figs. 17-4 and 17-5). The predominant malignant cells are usually small, of uneven size, round or elongated, with scanty cytoplasm and relatively large, hyperchromatic nuclei, wherein conspicuous nucleoli can often be seen. Elongated, spindle-form small malignant cells may also occur. These cells correspond to the sarcomatous component of these tumors, made up of small cells. Cells of coexisting carcinomas resemble those of endometrial carcinoma, squamous carcinoma, or both. Sometimes, carcinoma cells are the only malignant component observed in smears. More often, however, there is an association of elements of adeno- or squamous carcinoma with the small malignant cells described above, which is fairly characteristic of mesodermal mixed tumor. Cells of rhabdomyosarcoma, showing cytoplasmic cross-striations or at least markedly eosinophilic cytoplasm, are rarely seen. When they occur, however, they are diagnostic of rhabdomyosarcoma which, in most cases, is a component of a mesodermal mixed tumor (Fig. 17-6). Identifiable cells from other forms of sarcoma, such as chondrosarcoma, are exceedingly rare.
Mesodermal mixed tumor should be differentiated from endometrial or cervical carcinomas with undifferentiated components, which may be made up of spindly and giant tumor cells, thereby suggesting a co-existing sarcoma. Such tumors are often referred to as carcinosarcomas, but the name spindle-cell or spindle- and giant cell carcinoma appears more appropriate (see below). The prognosis of these tumors is better than that of mesodermal mixed tumors (Norris and Taylor, 1966; Mortel et al, 1974). The
separation of mesodermal mixed tumors from undifferentiated carcinomas in limited biopsy material may be very difficult.
separation of mesodermal mixed tumors from undifferentiated carcinomas in limited biopsy material may be very difficult.
Malignant Lymphomas
Current classification of malignant lymphomas is discussed in Chapter 31. Primary malignant lymphomas of the female genital tract are uncommon and only sporadic cases of such tumors occurring in the uterine cervix, vagina or ovary were recorded prior to 1980 (Johnson and Soule, 1957; Vieaux and McGuire, 1964; Iliya et al, 1968; Buchler and Kline, 1972; Katayama et al, 1973; Stransky et al, 1973; Delgado et al, 1976; Carr et al, 1976; Whitaker, 1976; Krumermann and Chung, 1978; Tunca et al, 1979). Within recent years, additional cases of primary malignant lymphomas of the uterine cervix have been reported (Komaki et al, 1984; Harris and Scully, 1984; Taki et al, 1985; Mann et al, 1987; Strang et al, 1988; Andrews et al, 1988; Perren et al, 1992; Clement, 1993; Gabriele and Gaudiano, 2003). We have personally observed several examples of malignant lymphoma of the uterine cervix and vagina. Vaginal bleeding may be the first manifestation of this group of diseases.
Cytology
The cytologic recognition of small-cell malignant lymphomas (or chronic lymphocytic leukemias which have identical presentation) in cervicovaginal smears is difficult. The cytologic samples contain a monotonous population of small lymphocytes without distinguishing features, except for granularity of the nuclei and irregularities of the nuclear contour. Young et al (1985) cautioned that benign inflammatory lymphoid infiltrates of the cervix, endometrium, and vulva may mimic malignant lymphomas. Lymphocytic cervicitis (see Chap. 10) is a case in point. The presence of polyclonal plasma cells and lymphocytes or polymorphonuclear leukocytes within the lymphocytic
lesion should be construed as a warning that the lesion may be inflammatory.
lesion should be construed as a warning that the lesion may be inflammatory.
The cytologic presentation of primary large-cell lymphomas is identical with that of secondary involvement (described in detail in Chaps. 26




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree