INTRODUCTION
Many different viruses can invade the central nervous system and cause disease. This chapter discusses rabies, a viral encephalitis feared since antiquity that is still an incurable disease; slow virus infections; and transmissible spongiform encephalopathies—rare neurodegenerative disorders that are caused by unconventional agents called “prions.”
RABIES
Rabies is an acute infection of the central nervous system that is almost always fatal. The virus is usually transmitted to humans from the bite of a rabid animal. Although the number of human cases is small, rabies is a major public health problem because it is widespread among animal reservoirs.
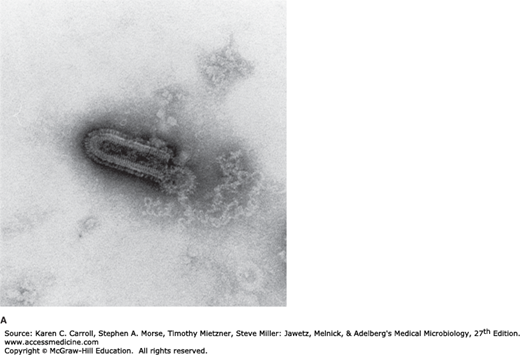
Rabies virus is a rhabdovirus with morphologic and biochemical properties in common with vesicular stomatitis virus of cattle and several animal, plant, and insect viruses (Table 42-1). The rhabdoviruses are rod- or bullet-shaped particles measuring 75 × 180 nm (Figure 42-1). The particles are surrounded by a membranous envelope with protruding spikes, 10 nm long. The peplomers (spikes) are composed of trimers of the viral glycoprotein. Inside the envelope is a ribonucleocapsid. The genome is single-stranded, negative-sense RNA (12 kb; molecular weight 4.6 × 106). Virions contain an RNA-dependent RNA polymerase. The particles have a buoyant density in CsCl of about 1.19 g/cm3 and a molecular weight of 300–1000 × 106.
| Virion: Bullet-shaped, 75 nm in diameter × 180 nm in length |
| Composition: RNA (4%), protein (67%), lipid (26%), carbohydrate (3%) |
| Genome: Single-stranded RNA, linear, nonsegmented, negative-sense, molecular weight 4.6 million, 12 kb |
| Proteins: Five major proteins; one is the envelope glycoprotein |
| Envelope: Present |
| Replication: Cytoplasm; virions bud from plasma membrane |
Outstanding characteristics: Wide array of viruses with broad host range Group includes the deadly rabies virus |
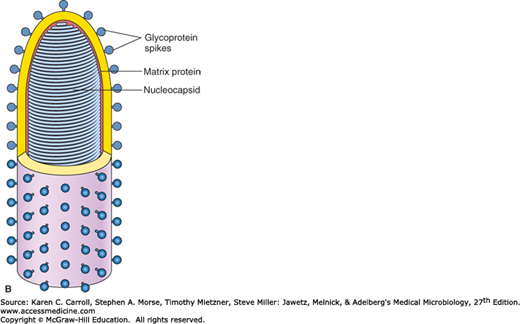
FIGURE 42-1
Structure of rhabdoviruses. A: Electron micrograph of bullet-shaped particle typical of the rhabdovirus family (100,000×). Shown here is vesicular stomatitis virus negatively stained with potassium phosphotungstate. (Courtesy of RM McCombs, M Benyesh-Melnick, and JP Brunschwig.) B: Schematic model of rabies virus showing the surface glycoprotein spikes extending from the lipid envelope that surrounds the internal nucleocapsid and the matrix protein lining the envelope. The nucleocapsid comprises the single RNA genome plus nucleoprotein and the polymerase proteins. (Reproduced with permission from Cowan MK, Talaro KP: Microbiology. A Systems Approach, 2nd ed. McGraw-Hill, 2009. © The McGraw-Hill Companies, Inc.)
The viruses are classified in the family Rhabdoviridae. Rabies viruses belong to the genus Lyssavirus, whereas the vesicular stomatitis-like viruses are members of the genus Vesiculovirus. The rhabdoviruses are very widely distributed in nature, infecting vertebrates, invertebrates, and plants. Rabies is the major medically important rhabdovirus. Many of the animal rhabdoviruses infect insects, but rabies virus does not.
Rabies virus survives storage at 4°C for weeks and at −70°C for years. It is inactivated by CO2, so on dry ice it must be stored in glass-sealed vials. Rabies virus is killed rapidly by exposure to ultraviolet radiation or sunlight, by heat (1 hour at 50°C), by lipid solvents (ether, 0.1% sodium deoxycholate), by trypsin, by detergents, and by extremes of pH.
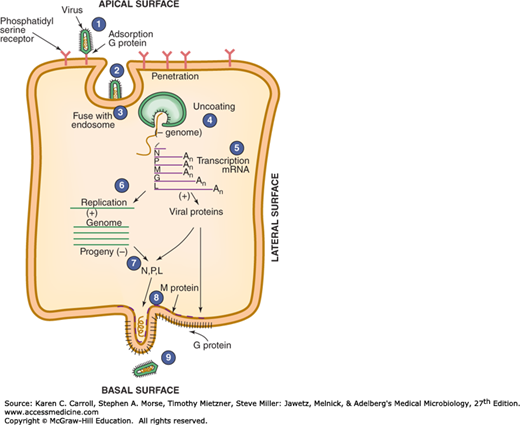
The rhabdovirus replication cycle is shown in Figure 42-2. Rabies virus attaches to cells via its glycoprotein spikes; the nicotinic acetylcholine receptor may serve as a cellular receptor for rabies virus. The single-stranded RNA genome is transcribed by the virion-associated RNA polymerase to five mRNA species. The template for transcription is the genome RNA in the form of ribonucleoprotein (RNP) (encased in N protein and containing the viral transcriptase). The monocistronic mRNAs code for the five virion proteins: nucleocapsid (N), polymerase proteins (L, P), matrix (M), and glycoprotein (G). The genome RNP is a template for complementary positive-sense RNA, which is responsible for the generation of negative-sense progeny RNA. The same viral proteins serve as polymerase for viral RNA replication as well as for transcription. Ongoing translation is required for replication, particularly of viral N and P proteins. The newly replicated genomic RNA associates with the viral transcriptase and nucleoprotein to form RNP cores in the cytoplasm. The particles acquire an envelope by budding through the plasma membrane. The viral matrix protein forms a layer on the inner side of the envelope, whereas the viral glycoprotein is on the outer layer and forms the spikes.
FIGURE 42-2
Steps in the replication of a rhabdovirus: (1) virus attachment; (2) penetration within an endosome; (3) fusion of virus with endosomal membrane, releasing core into cytoplasm; (4) uncoating of nucleocapsid; (5) viral negative-sense genomic RNA transcribed into positive-sense RNA; (6) positive-sense RNA serves as template for synthesis of viral genome, plus mRNA that gives rise to viral proteins; (7) negative-sense RNA becomes incorporated into nucleocapsids (N); (8) nucleocapsids join matrix protein (M) at cell surface; (9) budding of virus from cell surface. (Reproduced with permission from Levy JA, Fraenkel-Conrat H, Owens RA: Virology, 3rd ed. Prentice Hall, 1994.)
Rabies virus has a wide host range. All warm-blooded animals, including humans, can be infected. Susceptibility varies among mammalian species, ranging from very high (foxes, coyotes, wolves) to low (opossums); those with intermediate susceptibility include skunks, raccoons, and bats (Table 42-2). The virus is widely distributed in infected animals, especially in the nervous system, saliva, urine, lymph, milk, and blood. Recovery from infection is rare except in certain bats, where the virus has become peculiarly adapted to the salivary glands. Hematophagous vampire bats may transmit the virus for months without themselves ever showing any signs of disease.
When freshly isolated in the laboratory, the strains are referred to as street virus. Such strains show long and variable incubation periods (usually 21–60 days in dogs) and regularly produce intracytoplasmic inclusion bodies. Serial brain-to-brain passage in rabbits yields a “fixed” virus that no longer multiplies in extraneural tissues. This fixed (or mutant) virus multiplies rapidly, and the incubation period is shortened to 4–6 days. Inclusion bodies are found only with difficulty.
There is a single serotype of rabies virus. However, there are strain differences among viruses isolated from different species (raccoons, foxes, skunks, canines, bats) in different geographic areas. These viral strains can be distinguished by epitopes in the nucleoprotein and glycoprotein recognized by monoclonal antibodies as well as by specific nucleotide sequences. There are at least seven antigenic variants found in terrestrial animals and bats.
The G glycoprotein is a major factor in rabies virus neuroinvasiveness and pathogenicity. Avirulent mutants of rabies virus have been selected using certain monoclonal antibodies against the viral glycoprotein. A substitution at amino acid position 333 of the glycoprotein results in loss of virulence, indicating some essential role for that site of the protein in disease pathogenesis.
Purified spikes containing the viral glycoprotein elicit neutralizing antibody in animals. Antiserum prepared against the purified nucleocapsid is used in diagnostic immunofluorescence for rabies.
Rabies virus multiplies in muscle or connective tissue at the site of inoculation and then enters peripheral nerves at neuromuscular junctions and spreads up the nerves to the central nervous system. However, it is also possible for rabies virus to enter the nervous system directly without local replication. It multiplies in the central nervous system and progressive encephalitis develops. The virus then spreads through peripheral nerves to the salivary glands and other tissues. The organ with the highest titers of virus is the submaxillary salivary gland. Other organs where rabies virus has been found include pancreas, kidney, heart, retina, and cornea. Rabies virus has not been isolated from the blood of infected persons.
Susceptibility to infection and the incubation period may depend on the host’s age, genetic background, and immune status, the viral strain involved, the amount of inoculum, the severity of lacerations, and the distance the virus has to travel from its point of entry to the central nervous system. There is a higher attack rate and shorter incubation period in persons bitten on the face or head; the lowest mortality occurs in those bitten on the legs.
Rabies virus produces a specific eosinophilic cytoplasmic inclusion, the Negri body, in infected nerve cells. Negri bodies are filled with viral nucleocapsids. The presence of such inclusions is pathognomonic of rabies but is not observed in at least 20% of cases. Therefore, the absence of Negri bodies does not rule out rabies as a diagnosis. The importance of Negri bodies in rabies diagnosis has been lessened by the development of the more sensitive fluorescent antibody and reverse transcription-polymerase chain reaction diagnostic tests.
Rabies is primarily a disease of lower animals and is spread to humans by bites of rabid animals or by contact with saliva from rabid animals. The disease is an acute, fulminant, fatal encephalitis. The incubation period in humans is typically 1–3 months but may be as short as 1 week or more than a year. It is usually shorter in children than in adults. The clinical spectrum can be divided into three phases: a short prodromal phase, an acute neurologic phase, and coma. The prodrome, lasting 2–10 days, may show any of the following nonspecific symptoms: malaise, anorexia, headache, photophobia, nausea and vomiting, sore throat, and fever. Usually there is an abnormal sensation around the wound site.
During the acute neurologic phase, which lasts 2–7 days, patients show signs of nervous system dysfunction such as nervousness, apprehension, hallucinations, and bizarre behavior. General sympathetic overactivity is observed, including lacrimation, pupillary dilatation, and increased salivation and perspiration. A large fraction of patients will exhibit hydrophobia (fear of water) or aerophobia (fear when feeling a breeze). The act of swallowing precipitates a painful spasm of the throat muscles. This phase is followed by convulsive seizures or coma and death. The major cause of death is cardiorespiratory arrest. Paralytic rabies occurs in about 30% of patients, most frequently in those infected with bat rabies virus. The disease course is slower, with some patients surviving 30 days. Recovery and survival are extremely rare.
Rabies should be considered in any case of encephalitis or myelitis of unknown cause even in the absence of an exposure history, and particularly in a person who has lived or traveled outside the United States. Most cases of rabies in the United States are in individuals with no known exposure. Because of the long incubation period, people may forget a possible exposure incident. People who contract bat rabies often have no recollection of being bitten by a bat.
The usual incubation period in dogs ranges from 3 to 8 weeks, but it may be as short as 10 days. Clinically, the disease in dogs is divided into the same three phases as human rabies.
There are no tests to diagnose rabies infections in humans before the onset of clinical symptoms. Rabies can be diagnosed from euthanized animals by direct fluorescent antibody testing of brain tissue.
Tissues infected with rabies virus are currently identified most rapidly and accurately by means of immunofluorescence or immunoperoxidase staining using antirabies monoclonal antibodies. A biopsy specimen is usually taken from the skin of the neck at the hairline. Impression preparations of brain or cornea tissue may be used.
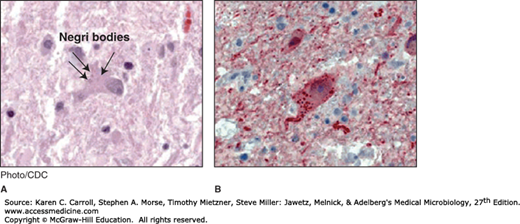
A definitive pathologic diagnosis of rabies can be based on the finding of Negri bodies in the brain or the spinal cord. They are sharply demarcated, more or less spherical, and 2–10 μm in diameter, and they have a distinctive internal structure with basophilic granules in an eosinophilic matrix. Negri bodies contain rabies virus antigens (Figure 42-3). Both Negri bodies and rabies antigen can usually be found in animals or humans infected with rabies, but they are rarely found in bats.
FIGURE 42-3
Histopathologic examination of central nervous system tissue from autopsy of a decedent with suspected rabies infection, showing neuronal cytoplasmic inclusions (Negri bodies) after hematoxylin and eosin staining (A) and rabies virus antigen (red) after immunohistochemical staining (B). (Source: Centers for Disease Control and Prevention: Human rabies—Kentucky/Indiana, 2009. MMWR Morb Mortal Wkly Rep 2010;59:393.)
Reverse transcription-polymerase chain reaction testing can be used to amplify parts of a rabies virus genome from fixed or unfixed brain tissue or saliva. Sequencing of amplified products can allow identification of the infecting virus strain.
Serum antibodies to rabies can be detected by immunofluorescence or neutralization tests. Such antibodies develop slowly in infected persons or animals during progression of the disease but promptly after vaccination with cell-derived vaccines. Antibodies in cerebrospinal fluid are produced in rabies-infected individuals but not in response to vaccination.