INTRODUCTION
The streptococci, enterococci, and related organisms are gram-positive spherical bacteria that characteristically form pairs or chains during growth. They are widely distributed in nature. Some are members of the normal human microbiota; others are associated with important human diseases attributable to the direct effects of infection or in other cases to an immunologic response to them. Streptococci elaborate a variety of extracellular substances and enzymes.
The streptococci are a large and heterogeneous group of bacteria, and no one system suffices to classify them. Yet, understanding their taxonomy is key to understanding their medical importance.
CLASSIFICATION OF STREPTOCOCCI
The classification of streptococci into major categories has been based on a series of observations over many years: (1) colony morphology and hemolytic reactions on blood agar, (2) serologic specificity of the cell wall group-specific substance (Lancefield antigens) and other cell wall or capsular antigens, (3) biochemical reactions and resistance to physical and chemical factors, and (4) ecologic features. More recently, molecular genetics have replaced phenotypic methods in the taxonomic assignment of these organisms. The classification of streptococci of medical importance is summarized in Table 14-1.
| Name | Group-Specific Substancea | Hemolysisb | Habitat | Important Laboratory Criteria | Common and Important Diseases |
|---|---|---|---|---|---|
| Pyogenic Streptococci | |||||
| Streptococcus pyogenes | A | β | Throat, skin | Large colonies (>0.5 mm), PYRc test positive, inhibited by bacitracin | Pharyngitis, impetigo, deep soft tissue infections; bacteremia; rheumatic fever, glomerulonephritis, toxic shock |
| Streptococcus agalactiae | B | β | Urogenital tract, lower GI tract | Hippurate hydrolysis, CAMP-factor positived | Neonatal sepsis and meningitis; bacteremia, UTIs,e meningitis in adults |
| Streptococcus dysgalactiae subspecies equisimilis; others | C, G | β (human) infections), α, none | Throat | Large (>0.5 mm) colonies | Pharyngitis, pyogenic infections similar to group A streptococci |
| Viridans Streptococci | |||||
| Streptococcus bovis groupf | D | None | Colon, biliary tree | Growth in presence of bile, hydrolyze esculin, no growth in 6.5% NaCl, degrades starch | Endocarditis, common blood isolate in colon cancer, biliary disease |
| Streptococcus anginosus group (S anginosus, Streptococcus intermedius, Streptococcus constellatus) | F (A, C, G) and untypeable | α, β, none | Throat, colon, urogenital tract | Small (<0.5 mm) colony variants of β-hemolytic species; group A are bacitracin resistant and PYR negative; carbohydrate fermentation patterns; arginine, esculin, VPg positive | Pyogenic infections, including brain, liver, lung abscesses |
| Mutans group | Usually not typed | α, none | Oral cavity | carbohydrate fermentation patterns; esculin, VP positive | Dental caries (S mutans), endocarditis; abscesses (with many other bacterial species) |
| Mitis-Sanguinis group | |||||
| Streptococcus pneumoniae | Noneº | α | Nasopharynx | Susceptible to optochin; colonies soluble in bile; quellung reaction positive | Pneumonia, meningitis, bacteremia, otitis media, sinusitis |
Streptococcus mitis | None | α, none | Oral cavity | VP negativeg; carbohydrate fermentation patterns | Endocarditis; bacteremia, sepsis in immunocompromised patients; high-level resistance to penicillin |
Salivarius group | None | α, none | Oral cavity | VP positive; carbohydrate fermentation patterns | Bacteremia, endocarditis, meningitis |
Many streptococci are able to hemolyze red blood cells in vitro in varying degrees. Complete disruption of erythrocytes with clearing of the blood around the bacterial growth is called β-hemolysis. Incomplete lysis of erythrocytes with reduction of hemoglobin and the formation of green pigment is called α-hemolysis. Other streptococci are nonhemolytic (sometimes called γ- [gamma-] hemolysis).
The hemolysis patterns of the streptococci of medical importance to humans are shown in Table 14-1. The classification of hemolytic patterns is used primarily with the streptococci although other bacteria that cause disease may also typically produce a variety of hemolysins.
This carbohydrate is contained in the cell wall of many streptococci and forms the basis of serologic grouping into Lancefield groups A–H and K–U. The serologic specificity of the group-specific carbohydrate is determined by an amino sugar. For group A streptococci, this is rhamnose-N-acetylglucosamine; for group B, it is rhamnose-glucosamine polysaccharide; for group C, it is rhamnose-N-acetylgalactosamine; for group D, it is glycerol teichoic acid containing d-alanine and glucose; and for group F, it is glucopyranosyl-N-acetylgalactosamine.
Extracts of group-specific antigen for grouping streptococci are prepared by a variety of methods, including extraction of centrifuged culture treated with hot hydrochloric acid, nitrous acid, or formamide; by enzymatic lysis of streptococcal cells (eg, with pepsin or trypsin); or by autoclaving of cell suspensions. These extracts contain the carbohydrate group–specific substance that yields precipitin reactions specific antisera. This permits arrangement of many streptococci into groups A–H and K–U. Typing is generally done only for groups A, B, C, F, and G (see Table 14-1), which cause disease in humans and for which reagents are available that allow typing using simple agglutination or color reactions.
The antigenic specificity of the capsular polysaccharides is used to classify Streptococcus pneumoniae into more than 90 types and to type the group B streptococci (Streptococcus agalactiae).
Biochemical tests include sugar fermentation reactions, tests for the presence of enzymes, and tests for susceptibility or resistance to certain chemical agents. Biochemical tests are most often used to classify streptococci after the colony growth and hemolytic characteristics have been observed. Biochemical tests are used for species that typically do not react with the commonly used antibody preparations for the group-specific substances, groups A, B, C, F, and G. For example, the viridans streptococci are α-hemolytic or nonhemolytic and do not react with the antibodies commonly used for the Lancefield classification. Speciation of the viridans streptococci requires a battery of biochemical tests. See Table 14-1. However, because biochemical reactions are labor intensive and often unreliable, laboratories with molecular capabilities, such as gene sequencing or that have implemented mass spectrometry for organism identification (matrix-assisted laser desorption ionization-time of flight mass spectrometry [MALDI-TOF MS]), are replacing phenotypic tests with these methods when identification of viridians streptococci is required.
STREPTOCOCCI OF PARTICULAR MEDICAL INTEREST
The following streptococci and enterococci are of particular medical relevance.
Most streptococci that contain the group A antigen are S pyogenes. It is a prototypical human pathogen. It is used here to illustrate general characteristics of streptococci and specific characteristics of the species. S pyogenes is the main human pathogen associated with local or systemic invasion and poststreptococcal immunologic disorders. S pyogenes typically produces large (1 cm in diameter) zones of β-hemolysis around colonies greater than 0.5 mm in diameter. They are PYR-positive (hydrolysis of l-pyrrolidonyl-β-naphthylamide) and usually are susceptible to bacitracin.
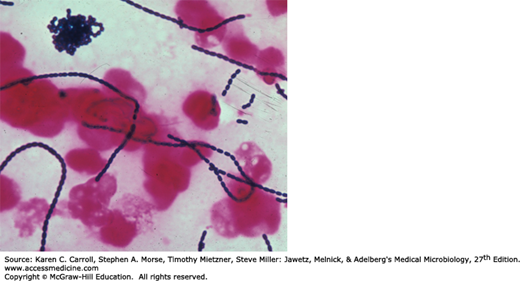
Individual cocci are spherical or ovoid and are arranged in chains (Figure 14-1). The cocci divide in a plane perpendicular to the long axis of the chain. The members of the chain often have a striking diplococcal appearance, and rod-like forms are occasionally seen. The lengths of the chains vary widely and are conditioned by environmental factors. Streptococci are gram positive; however, as a culture ages and the bacteria die, they lose their gram positivity and can appear to be gram negative; for some streptococci, this can occur after overnight incubation.
Most group A strains (see Table 14-1) produce capsules composed of hyaluronic acid. The capsules are most noticeable in very young cultures. They impede phagocytosis. The hyaluronic acid capsule likely plays a greater role in virulence than is generally appreciated and together with M protein was believed to be an important factor in the resurgence of rheumatic fever (RF) in the United States in the 1980s and 1990s. The capsule binds to hyaluronic-acid-binding protein, CD44, present on human epithelial cells. Binding induces disruption of intercellular junctions allowing microorganisms to remain extracellular as they penetrate the epithelium (see Stollerman and Dale, 2008). Capsules of other streptococci (eg, S agalactiae and S pneumoniae) are different. The S pyogenes cell wall contains proteins (M, T, R antigens), carbohydrates (group specific), and peptidoglycans. Hairlike pili project through the capsule of group A streptococci. The pili consist partly of M protein and are covered with lipoteichoic acid. The latter is important in the attachment of streptococci to epithelial cells.
Most streptococci grow in solid media as discoid colonies, usually 1–2 mm in diameter. S pyogenes is β-hemolytic (Figure 14-2); other species have variable hemolytic characteristics (see Table 14-1).
Energy is obtained principally from the utilization of glucose with lactic acid as the end product. Growth of streptococci tends to be poor on solid media or in broth unless enriched with blood or tissue fluids. Nutritive requirements vary widely among different species. The human pathogens are most exacting, requiring a variety of growth factors. Growth and hemolysis are aided by incubation in 10% CO2. Most pathogenic hemolytic streptococci grow best at 37°C. Most streptococci are facultative anaerobes and grow under aerobic and anaerobic conditions.
Variants of the same Streptococcus strain may show different colony forms. This is particularly marked among S pyogenes strains, giving rise to either matte or glossy colonies. Matte colonies consist of organisms that produce much M protein and generally are virulent. The S pyogenes in glossy colonies tend to produce little M protein and are often not virulent.
This substance is a major virulence factor of S pyogenes. M protein is a filamentous structure anchored to the cell membrane that penetrates and projects from the streptococcal cell wall. When M protein is present, the streptococci are virulent, and in the absence of M type-specific antibodies, they are able to resist phagocytosis by polymorphonuclear leukocytes by inhibiting activation of the alternate complement pathway. S pyogenes that lack M protein are not virulent. Immunity to infection with group A streptococci is related to the presence of type-specific antibodies to M protein. Because there are more than 150 types of M protein, a person can have repeated infections with S pyogenes of different M types. Both groups C and G streptococci have genes homologous to the genes for M protein of group A, and M proteins similar to those of group A have been found on groups C and G streptococci.
The M protein molecule has a rodlike coiled structure that separates functional domains. The structure allows for a large number of sequence changes while maintaining function, and the M protein immunodeterminants, therefore, can readily change. There are two major structural classes of M protein, classes I and II.
It appears that M protein and perhaps other streptococcal cell wall antigens have an important role in the pathogenesis of rheumatic fever. Purified streptococcal cell wall membranes induce antibodies that react with human cardiac sarcolemma; the characteristics of the cross-reactive antigens are not clear. A component of the cell wall of selected M types induces antibodies that react with cardiac muscle tissue. Conserved antigenic domains on the class I M protein cross-react with human cardiac muscle, and the class I M protein may be a virulence determinant for rheumatic fever.
More than 20 extracellular products that are antigenic are elaborated by S pyogenes, including the following.
Streptokinase is produced by many strains of group A β-hemolytic streptococci. It transforms the plasminogen of human plasma into plasmin, an active proteolytic enzyme that digests fibrin and other proteins, allowing the bacteria to escape from blood clots. This process of digestion may be interfered with by nonspecific serum inhibitors and by a specific antibody, antistreptokinase. Streptokinase has been given intravenously for treatment of pulmonary emboli, coronary artery, and venous thromboses.
Streptococcal deoxyribonucleases A, B, C, and D degrade DNA (DNases) and similar to streptokinase facilitate the spread of streptococci in tissue by liquefying pus. The enzymatic activity can be measured by the decrease in viscosity of known DNA solutions. Purulent exudates owe their viscosity largely to deoxyribonucleoprotein. Mixtures of streptokinase and DNases are used in “enzymatic debridement.” They help to liquefy exudates and facilitate removal of pus and necrotic tissue; antimicrobial drugs thus gain better access, and infected surfaces recover more quickly. An antibody to DNAse develops after streptococcal infections (normal limit, 100 units), especially after skin infections.
Hyaluronidase splits hyaluronic acid, an important component of the ground substance of connective tissue. Thus, hyaluronidase aids in spreading infecting microorganisms (spreading factor). Hyaluronidases are antigenic and specific for each bacterial or tissue source. After infection with hyaluronidase-producing organisms, specific antibodies are found in the serum.
Pyrogenic exotoxins are elaborated by S pyogenes. There are three antigenically distinct streptococcal pyrogenic exotoxins (Spe): A, B, and C. SpeA has been most widely studied. It is produced by group A streptococci that carry a lysogenic phage. The streptococcal pyrogenic exotoxins have been associated with streptococcal toxic shock syndrome and scarlet fever. Most strains of group A streptococci isolated from patients with streptococcal toxic shock syndrome either produce Spe A or have the gene that codes for it; in contrast, only about 15% of group A streptococci isolated from other patients have the gene. Spe C, also encoded by a phage, may contribute to the syndrome. Spe B, a potent protease, interferes with phagocytosis. The group A streptococci associated with toxic shock syndrome are primarily of M protein types 1 and 3.
The pyrogenic exotoxins act as superantigens, which stimulate T cells by binding to the class II major histocompatibility complex in the Vβ region of the T-cell receptor. The activated T cells release cytokines that mediate shock and tissue injury. The mechanisms of action appear to be similar to those caused by staphylococcal toxic shock syndrome toxin-1 and the staphylococcal enterotoxins.
The β-hemolytic group A S pyogenes elaborates two hemolysins (streptolysins) that not only lyse the membranes of erythrocytes but also damage a variety of other cell types. Streptolysin O is a protein (molecular weight [MW], 60,000) that is hemolytically active in the reduced state (available–SH groups) but rapidly inactivated in the presence of oxygen. Streptolysin O is responsible for some of the hemolysis seen when growth occurs in cuts made deep into the medium in blood agar plates. It combines quantitatively with antistreptolysin O (ASO), an antibody that appears in humans after infection with any streptococci that produce streptolysin O. This antibody blocks hemolysis by streptolysin O. This phenomenon forms the basis of a quantitative test for the antibody. An ASO serum titer in excess of 160–200 units is considered abnormally high and suggests either recent infection with S pyogenes or persistently high antibody levels caused by an exaggerated immune response to an earlier exposure in a hypersensitive person. Streptolysin S is the agent responsible for the hemolytic zones around streptococcal colonies growing on the surface of blood agar plates. It is elaborated in the presence of serum—hence the name streptolysin S. It is not antigenic. Most isolates of S pyogenes produce both of these hemolysins. Up to 10% produce only one.
A variety of distinct disease processes are associated with S pyogenes infections. The infections can be divided into several categories.
The portal of entry determines the principal clinical picture. In each case, however, there is a diffuse and rapidly spreading infection that involves the tissues and extends along lymphatic pathways with only minimal local suppuration. From the lymphatics, the infection can extend to the bloodstream.
1. Erysipelas—If the portal of entry is the skin, erysipelas results. Lesions are raised and characteristically red. There is massive brawny edema and a rapidly advancing, sharply demarcated margin of infection.
2. Cellulitis—Streptococcal cellulitis is an acute, rapidly spreading infection of the skin and subcutaneous tissues. It follows infection associated with mild trauma, burns, wounds, or surgical incisions. Pain, tenderness, swelling, and erythema occur. Cellulitis is differentiated from erysipelas by two clinical findings: In cellulitis, the lesion is not raised, and the line between the involved and uninvolved tissue is indistinct.
3. Necrotizing fasciitis (streptococcal gangrene)—There is extensive and very rapidly spreading necrosis of the skin, tissues, and fascia. Bacteria other than S pyogenes can also cause necrotizing fasciitis. The group A streptococci that cause necrotizing fasciitis have sometimes been termed flesh-eating bacteria.
4. Puerperal fever—If the streptococci enter the uterus after delivery, puerperal fever develops, which is essentially a septicemia originating in the infected wound (endometritis).
5. Bacteremia or sepsis—Infection of traumatic or surgical wounds with streptococci results in bacteremia, which can rapidly be fatal. S pyogenes bacteremia can also occur with skin infections, such as cellulitis and rarely pharyngitis.
1. Streptococcal sore throat—The most common infection caused by β-hemolytic S pyogenes is streptococcal sore throat or pharyngitis. S pyogenes adheres to the pharyngeal epithelium by means of lipoteichoic acid–covered surface pili and by means of hyaluronic acid in encapsulated strains. The glycoprotein fibronectin (MW, 440,000) on epithelial cells probably serves as lipoteichoic acid ligand. In infants and small children, the sore throat occurs as a subacute nasopharyngitis with a thin serous discharge and little fever but with a tendency of the infection to extend to the middle ear and the mastoid. The cervical lymph nodes are usually enlarged. The illness may persist for weeks. In older children and adults, the disease is more acute and is characterized by intense nasopharyngitis, tonsillitis, and intense redness and edema of the mucous membranes, with purulent exudate; enlarged, tender cervical lymph nodes; and (usually) a high fever. Twenty percent of infections are asymptomatic. A similar clinical picture can occur with infectious mononucleosis, diphtheria, gonococcal infection, and adenovirus infection.
S pyogenes infection of the upper respiratory tract does not usually involve the lungs. Pneumonia, when it does occur, is rapidly progressive and severe and is most commonly a sequela to viral infections, such as influenza or measles, which seem to greatly enhance the predisposition to bacterial superinfection with this and other pathogens, such as S pneumoniae.
2. Streptococcal pyoderma—Local infection of superficial layers of skin, especially in children, is called impetigo. It consists of superficial vesicles that break down and eroded areas whose denuded surface is covered with pus and later is encrusted. It spreads by continuity and is highly communicable, especially in hot, humid climates. More widespread infection occurs in eczematous or wounded skin or in burns and may progress to cellulitis. Group A streptococcal skin infections are often attributable to M types 49, 57, and 59–61 and may precede glomerulonephritis (GN) but do not lead to rheumatic fever.
A clinically identical infection can be caused by Staphylococcus aureus and sometimes both S pyogenes and S aureus are present.
Fulminant, invasive S pyogenes infections with streptococcal toxic shock syndrome are characterized by shock, bacteremia, respiratory failure, and multiorgan failure. Death occurs in about 30% of patients. The infections tend to occur after minor trauma in otherwise healthy persons with several presentations of soft tissue infection. These include necrotizing fasciitis, myositis, and infections at other soft tissue sites; bacteremia occurs frequently. In some patients, particularly those infected with group A streptococci of M types 1 or 3, the disease presents with focal soft tissue infection accompanied by fever and rapidly progressive shock with multiorgan failure. Erythema and desquamation may occur. The S pyogenes of the M types 1 and 3 (and types 12 and 28) that make pyrogenic exotoxin A or B are associated with the severe infections.
Pyrogenic exotoxins A–C also cause scarlet fever in association with S pyogenes pharyngitis or with skin or soft tissue infection. The pharyngitis may be severe. The rash appears on the trunk after 24 hours of illness and spreads to involve the extremities. Streptococcal toxic shock syndrome and scarlet fever are clinically overlapping diseases.
After an acute S pyogenes infection, there is a latent period of 1–4 weeks (mean 7 days), after which nephritis or rheumatic fever occasionally develops. The latent period suggests that these poststreptococcal diseases are not attributable to the direct effect of disseminated bacteria but instead represent a hypersensitivity response. Nephritis is more commonly preceded by infection of the skin; rheumatic fever is more commonly preceded by infection of the respiratory tract.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree