INTRODUCTION
This chapter offers a brief survey of the protozoan and helminthic parasites of medical importance. A synopsis of each parasite is provided within tables that are organized by the organ system that is infected (eg, intestinal and blood/tissue protozoan infections and intestinal and blood/tissue helminthic infections). Key concepts are provided at the beginning of the protozoa and helminths sections to give the reader an overview of the paradigms in medical parasitology. Current updates to information provided in this chapter can be found at the Centers for Disease Control and Prevention (CDC) website www.cdc.gov/ncidod/dpd.
CLASSIFICATION OF PARASITES
The parasites covered in this chapter are categorized into two major groups: parasitic protozoa and parasitic helminths.
Protozoa are unicellular eukaryotes that form an entire kingdom. Classifying protozoan parasites into taxonomic groups is an ongoing process, and their status is often in a state of flux. For this reason, this chapter separates the parasitic protozoa into four traditional groups based on their means of locomotion and mode of reproduction: flagellates, amebae, sporozoa, and ciliates. Table 46-1 lists several medically important protozoan parasites by the organ system they infect, the mode of infection, diagnosis, treatment, and geographic location.
| Parasite/Disease | Site of Infection | Mechanism of Infection | Diagnosis | Treatment | Geographic Area |
|---|---|---|---|---|---|
| Intestinal protozoa | |||||
Giardia lamblia (flagellate) Giardiasis | Small intestine | Ingest cysts in water, not killed by normal chlorination | Stool exam for O&P; EIA for antigens | Metronidazole or nitazoxanide | Ubiquitous: campers, ski resorts, dogs, wild animals, especially beavers |
Entamoeba histolytica (ameba) Amebiasis | Colon; liver; other organs | Ingest cysts from fecal contamination of water or food or oral/anal behaviors | Stool exam for O&P; EIA for antibodies and antigen | Iodoquinol, or paromomycin | Worldwide wherever fecal contamination occurs |
Cryptosporidium (sporozoa) Cryptosporidiosis | Small intestine; respiratory tract | Ingest oocysts, fecal contamination | Stool exam/acid-fast staining; direct-fluorescent staining; EIA for antigens | Nitazoxanide for immunocompetent | Ubiquitous, especially in cattle-raising areas |
Cyclospora (sporozoa) Cyclosporiasis | Small intestine | Oocysts from fecal contamination of water, fresh produce | Stool exam—acid-fast staining, UV fluorescence microscopy | Trimethoprim/sulfamethoxazole | Worldwide, tropics, subtropics |
| Sexually transmitted protozoa | |||||
Trichomonas vaginalis (flagellate) Trichomoniasis | Vagina; males usually asymptomatic | Trophozoites passed from person to person through sexual intercourse | Microscopic exam of discharge, urine, tissue scraping | Metronidazole for both partners | Ubiquitous in sexually active populations |
| Blood and tissue flagellates | |||||
Trypanosoma brucei rhodesiense East African trypanosomiasis, sleeping sickness | Blood, lymph | Tsetse bite (painful) lacerates skin and releases trypomastigotes | Trypomastigotes (extracellular) in blood smear, CSF, or lymph node aspirate; serology (CATT) | Hemolytic stage: Suramin Late CNS involvement: Melarsoprol | East Africa; antelope, bushbuck are animal reservoirs for human infection |
Trypanosoma brucei gambiense West African trypanosomiasis, sleeping sickness | Blood, lymph | Tsetse bite (painful) lacerates skin and releases trypomastigotes | Trypomastigotes (extracellular) in blood smear, CSF, or lymph node aspirate; serology (CATT) | Hemolytic stage: pentamidine Late CNS involvement: Eflornithine | West Africa; vegetation around rivers; humans only (not zoonotic) |
| Trypanosoma cruzi Chagas disease | Amastigotes intracellular; heart, parasympathetic ganglia | Kissing bug feces rubbed into bite or eye; blood transfusion; transplacental transmission | Trypomastigotes (extracellular) in blood smear; PCR; intracellular amastigotes in tissue bx | Benznidazole | North, Central, and South America (bugs live in thatched roofs, mud cracks) |
Leishmania major Leishmania tropica cutaneous leishmaniasis | Skin; rolled edge ulceration | Sandfly injects promastigotes; amastigotes in macrophages, monocytes | Skin bx at edge of ulcer; histopathology; culture and PCR of organisms; intradermal leishmanin (Montenegro) skin test | Stibogluconate sodium, meglumine antimonate, pentamidine | Mid-East, India, North Africa |
Leishmania mexicana complex cutaneous leishmaniasis | Skin; rolled edge ulceration | Sandfly injects promastigotes; amastigotes in macrophages, monocytes | Skin bx at edge of ulcer; histopathology; culture and PCR of organisms; intradermal leishmanin (Montenegro) skin test | Stibogluconate sodium, meglumine antimonate, pentamidine | Mexico, Central and South America; chiclero ulcers on ears of chicle harvesters in Yucatan |
Leishmania aethiopica, Leishmania mexicana pifanoi Disseminated or diffuse form of cutaneous leishmaniasis | Skin; anergy resulting in nonulcerating lesions over entire body | Sandfly injects promastigotes; amastigotes in macrophages, monocytes | Skin bx at edge of ulcer; histopathology; culture and PCR of organisms; intradermal leishmanin (Montenegro) skin test | Sodium stibogluconate, meglumine antimonate, pentamidine | Ethiopia, Venezuela |
Leishmania brasiliensis complex Mucocutaneous leishmaniasis | Skin lesion; may destroy mucocutaneous tissues on face, mouth | Sandfly injects promastigotes; amastigotes in macrophages, monocytes | Skin bx at edge of ulcer; histopathology; culture and PCR of organisms; intradermal leishmanin (Montenegro) skin test | Sodium stibogluconate, meglumine antimonite, amphotericin B | Brazil, Peru, Bolivia |
Leishmaniasis donovani Kala-azar, visceral leishmaniasis | Sandfly injects promastigotes; amastigotes in macrophages and monocytes of spleen, liver, bone marrow | Bx spleen, liver, bone marrow aspirate; histopathology; culture and PCR of organisms | Liposomal amphotericin B, sodium stibogluconate, meglumine antimonite, amphotericin B | Post-kala-azar dermal leishmaniasis 1–3 years after Rx India, Sudan, South Sudan, Ethiopia, Kenya, Brazil | |
| Tissue amebae | |||||
Naegleria, Acanthamoeba, Balamuthia Primary amebic meningoencephalitis (Entamoeba histolytica—amebiasis, see intestinal protozoa) | Brain, spinal cord, eye | Swimming in warm freshwater lakes, ponds, rivers, hot springs; free-living amebae enter nasal membrane, pass to brain or via wound or penetration of eye (Acanthamoeba) | Trophozoite in CSF; clinical suspicion based on recent history of swimming or diving in warm waters | Amphotericin B | Where free-living amebae survive in sediment of warm fresh waters |
| Blood and tissue sporozoa | |||||
| Plasmodium vivax malaria | Intracellular in RBCs; hypnozoites in liver can cause relapse | Female Anopheles mosquito releases sporozoites into bloodstream; parasites enter liver, then blood; can relapse | Thick and thin blood smears; ring stage in RBCs with Schüffner dots; RDTs | aUncomplicated vivax: chloroquine plus primaquine (where no resistance), otherwise quinine plus doxycycline or tetracycline plus primaquine for relapse | Mostly Asia, Latin America, some areas of Africa (rare in west Africa) |
Plasmodium falciparum Malaria | Intracellular in RBCs | Female Anopheles mosquito releases sporozoites into bloodstream; parasites enter liver, then blood; no relapse | Thick and thin blood smears; banana-shaped gametocytes; double rings in RBCs; RDTs | aUncomplicated falciparum: chloroquine (where no resistance), otherwise artemether/lumefantrine (Coartem, artemisinin-based combination therapy, ACT) | Worldwide in tropical and subtropical areas |
Plasmodium ovale Malaria | Intracellular in RBCs; hypnozoites in liver can cause relapse | Female Anopheles mosquito releases sporozoites into bloodstream; parasites enter liver, then blood; can relapse | Thick and thin blood smears | aUncomplicated malaria: chloroquine (where no resistance); primaquine for relapse | Mostly Africa, especially West Africa, Islands of western Pacific |
Plasmodium malariae Malaria | Intracellular in RBCs; hypnozoites in liver can cause relapse | Enters liver from inoculation into bloodstream by infected mosquito; no relapse | Thick and thin blood smears | Chloroquine (where no resistance) | Worldwide |
Plasmodium knowlesi Primate malaria | Intracellular in RBCs | Female Anopheles mosquito releases sporozoites into bloodstream; parasites enter liver, then blood; hypnozoites not yet found | Thick and thin blood smears | Chloroquine (where no resistance) | Southeast Asia |
| Babesia microtiBabesiosis | Intracellular in RBCs | Tick bite; blood transfusions | Blood smears; tetrad forms (“Maltese Cross”) inside RBCs | Clindamycin plus quinine; atovaquone plus azithromycin | USA, Europe |
| Toxoplasma gondii Toxoplasmosis | Intracellular in CNS, bone marrow | Ingestion of parasites in undercooked meat; ingestion of oocysts from cat feces; transplacental; blood transfusion | Serology (IgG and IgM) | Pyrimethamine plus sulfadiazine | Worldwide; areas where cats/felids live |
(1) Flagellates have one or more whiplike flagella and, in some cases, an undulating membrane (eg, trypanosomes). These include intestinal and genitourinary flagellates (Giardia and Trichomonas, respectively) and blood and tissue flagellates (Trypanosoma and Leishmania). (2) Amebae are typically ameboid and use pseudopodia or protoplasmic flow to move. They are represented in humans by species of Entamoeba, Naegleria, and Acanthamoeba. (3) Sporozoa undergo a complex life cycle with alternating sexual and asexual reproductive phases. The human parasites Cryptosporidium, Cyclospora, and Toxoplasma and the malarial parasites (Plasmodium species) are all intracellular parasites. (4) Ciliates are complex protozoa bearing cilia distributed in rows or patches, with two kinds of nuclei in each individual. Balantidium coli, a giant intestinal ciliate of humans and pigs, is the only human parasite representative of this group, and because the disease is considered rare, it is not covered in this chapter.
Formerly listed with the sporozoa, because they possess polar filaments within a spore, microsporidia include more than 1000 species of intracellular parasites that infect invertebrates (mostly insects) and vertebrate hosts. In humans, microsporidians are opportunistic parasites of immunocompromised patients, including those undergoing chemotherapy and organ transplants.
Pneumocystis jiroveci was long considered a protozoan parasite but has been shown to be a member of the fungi rather than the protozoa. It causes interstitial plasma cell pneumonitis in immunosuppressed individuals and is considered an opportunistic pathogen.
Parasitic helminths, or worms of humans, belong to two phyla: Nematoda (roundworms) and Platyhelminthes (flatworms).
(1) Nematodes are among the most speciose and diverse animals. They are elongated and tapered at both ends, round in cross-section, and unsegmented. They have only a set of longitudinal muscles, which allows them to move in a whiplike, penetrating fashion; a complete digestive system that is well adapted for ingestion of the host’s gut contents, cells, blood, or cellular breakdown products; and a highly developed separate-sexed reproductive system. They shed their tough cuticles (molt) as they undergo development from larvae to adults, and the eggs and larval stages are well suited for survival in the external environment. Most human infections are acquired by ingestion of the egg or larval stage, but nematode infections can also be acquired from insect vectors and skin penetration. (2) Platyhelminthes are flatworms that are dorsoventrally flattened in cross-section and are hermaphroditic, with a few exceptions. All medically important species belong to two classes: Trematoda (flukes) and Cestoda (tapeworms).
Trematodes are typically flattened and leaf shaped with two muscular suckers. They have a bifurcated gut and possess both circular and longitudinal muscles; they lack the cuticle characteristic of nematodes and instead have a syncytial epithelium. Trematodes are hermaphroditic, with the exception of the schistosomes (blood flukes), which have male and female worms that exist coupled together within small blood vessels of their hosts.
The life cycle of human trematodes is typically initiated when eggs are passed into fresh water via feces or urine. Eggs develop, hatch, and release a ciliated miracidium, which infects a snail host that is usually highly specific to the fluke species. Within the snail, the miracidium develops into a sporocyst, which contains germinal cells that ultimately develop into the final larval stage—the cercariae. These swim out of the snail and encyst as metacercariae in a second intermediate host or on vegetation, depending on the species. Most fluke infections are acquired by ingestion of the metacercariae. The cercariae of schistosomes, however, directly penetrate the skin of their hosts and do not encyst as metacercariae.
Cestodes, or tapeworms, are flat and have a ribbon-like chain of segments (proglottids) containing male and female reproductive structures. Adult tapeworms can reach lengths of 10 m and have hundreds of segments, with each segment releasing thousands of eggs. At the anterior end of an adult tapeworm is the scolex, which is often elaborated with muscular suckers, hooks, or structures that aid in its ability to attach to the intestinal wall. Adult tapeworms have no mouth or gut and absorb their nutrients directly from their host through their integument.
The life cycle of cestodes, like that of the trematodes, is usually indirect (involving one or more intermediate hosts and a final host). Eggs are excreted with the feces and ingested by an intermediate host (invertebrate, such as a flea, or vertebrate, such as a mammal); the larvae develop into certain forms that are peculiar to the specific species within the intermediate host (eg, cysticercus in the case of Taenia solium or hydatid cyst with Echinococcus granulosus). Cestode larvae are generally eaten, and the larva develops into an adult worm in the intestine of the final host.
INTESTINAL PROTOZOAN INFECTIONS
Key concepts pertaining to parasitic protozoa and the protozoa included in this chapter are listed in Tables 46-2 and 46-3. A synopsis of the parasitic protozoan infections is provided in Table 46-1.
| Parasitic protozoa covered in this chapter are grouped into the flagellates, amebae, sporozoa, and ciliates. |
| Flagellates and amebae multiply by binary fission; sporozoans reproduce by a process known as merogony (also called schizogony) in which the nuclei replicate prior to cytokinesis. |
| Sporozoans (Cryptosporidium, Plasmodium, Toxoplasma) undergo sexual recombination, which leads to genomic and antigenic variation. |
| Protozoa can multiply quickly (on the order of several hours) in the host and can cause a rapid onset of symptoms. |
| Intestinal infections are acquired by ingestion of an environmentally resistant cyst (or oocyst) form; blood infections are vectorborne. |
| Infections by intracellular protozoa (Trypanosoma cruzi, Leishmania spp., Cryptosporidium, Toxoplasma, and Plasmodium) are difficult to treat because drugs must cross plasma membranes. No vaccines are available for any human parasitic disease. |
| Latent infections occur with Toxoplasma (parasites in tissue cysts are called bradyzoites) and Plasmodium vivax and Plasmodium ovale (parasites in liver tissue are called hypnozoites). |
| In disseminated protozoal infections, fever and flulike symptoms occur and are nonspecific. |
| Some parasitic protozoa are able to evade the host’s immune response because they are intracellular and/or undergo antigenic variation. |
| Intestinal protozoa |
| Giardia lamblia (flagellate) |
| Entamoeba histolytica (ameba) |
| Cryptosporidium hominis (sporozoa) |
| Cyclospora cayetanensis (sporozoa) |
| Sexually transmitted protozoan infection |
| Trichomonas vaginalis (flagellate) |
| Blood and tissue protozoan infections |
| Flagellates |
| Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense |
| Trypanosoma cruzi |
| Leishmania donovani, Leishmania tropica, Leishmania mexicana |
| Amebae |
| Entamoeba histolytica (see intestinal protozoa) |
| Naegleria fowleri and Acanthamoeba castellanii |
| Sporozoa |
| Plasmodium vivax, Plasmodium falciparum, Plasmodium ovale, and Plasmodium malariae |
| Babesia microti |
| Toxoplasma gondii |
| Microsporidia |
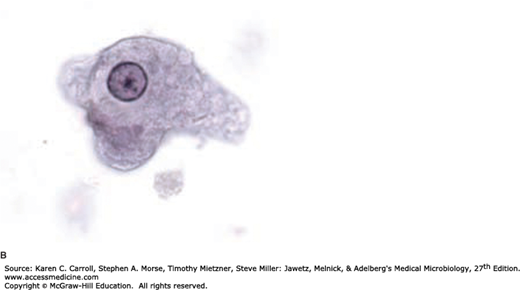
Giardia lamblia (also referred to as Giardia duodenalis or Giardia intestinalis) is the causative agent of giardiasis and is the only common pathogenic protozoan found in the duodenum and jejunum of humans. Giardia exists in two forms: the trophozoite and the cyst forms. The trophozoite of G lamblia is a heart-shaped organism, has four pairs of flagella, and is approximately 15 μm in length (Figure 46-1A). A large concave sucking disk on the ventral surface helps the organism to adhere to intestinal villi. As the parasites pass into the colon, they typically encyst, and the cysts are passed in the stool (Figure 46-1B). They are ellipsoid, thick-walled, highly resistant, and 8–14 μm in length; they contain two nuclei as immature forms and four as mature cysts.
G lamblia is usually only weakly pathogenic for humans. Cysts may be found in large numbers in the stools of entirely asymptomatic persons. In some persons, however, large numbers of parasites attached to the bowel wall may cause irritation and low-grade inflammation of the duodenal or jejunal mucosa, with consequent acute or chronic diarrhea associated with crypt hypertrophy, villous atrophy or flattening, and epithelial cell damage. Stools may be watery, semisolid, greasy, bulky, and foul smelling at various times during the course of the infection. Symptoms of malaise, weakness, weight loss, abdominal cramps, distention, and flatulence may continue for long periods. Collecting multiple stool samples over several days is recommended to increase the likelihood of microscopically detecting cysts in smears.
G lamblia occurs worldwide. Humans are infected by ingestion of fecally contaminated water or food containing giardia cysts or by direct fecal contamination, as may occur in day care centers, refugee camps, and institutions, or during oral–anal sex. Epidemic outbreaks have been reported at ski resorts in the United States, where overloading of sewage facilities or contamination of the water supply has resulted in sudden outbreaks of giardiasis. Cysts can survive in water for up to 3 months. Outbreaks among campers in wilderness areas suggest that humans may be infected with various animal giardia harbored by rodents, deer, cattle, sheep, horses, or household pets.
Entamoeba histolytica cysts are present only in the lumen of the colon and in mushy or formed feces and range in size from 10 to 20 μm (Figure 46-2A). The cyst may contain a glycogen vacuole and chromatoid bodies (masses of ribonucleoprotein) with characteristic rounded ends (in contrast to splinter chromatoidals in developing cysts of Entamoeba coli). Nuclear division occurs within the cyst, resulting in a quadrinucleated cyst, and the chromatoid bodies and glycogen vacuoles disappear. Diagnosis in most cases rests on the characteristics of the cyst, as trophozoites usually appear only in diarrheic feces in active cases and survive for only a few hours.
The ameboid trophozoite is the only form present in tissues (Figure 46-2B). The cytoplasm has two zones, a hyaline outer margin and a granular inner region that may contain red blood cells (pathognomonic) but ordinarily contains no bacteria. The nuclear membrane is lined by fine, regular granules of chromatin with a small central body (endosome or karyosome).
It is estimated that approximately 50 million cases of invasive disease occur each year, with up to 100,000 deaths (Marie and Petri, 2014). Disease results when the trophozoites of E histolytica invade the intestinal epithelium and form discrete ulcers with a pinhead-sized center and raised edges, from which mucus, necrotic cells, and amebae pass. The trophozoites multiply and accumulate above the muscularis mucosae, often spreading laterally. Rapid lateral spread of the multiplying amebae follows, undermining the mucosa and producing the characteristic “flask-shaped” ulcer of primary amebiasis: a small point of entry, leading via a narrow neck through the mucosa into an expanded necrotic area in the submucosa. Bacterial invasion usually does not occur at this time, cellular reaction is limited, and damage is by lytic necrosis.
Subsequent spread may coalesce colonies of amebae, undermining large areas of the mucosal surface. Trophozoites may penetrate the muscle layers and occasionally the serosa, leading to perforation into the peritoneal cavity. Subsequent enlargement of the necrotic area produces gross changes in the ulcer, which may develop shaggy overhanging edges, secondary bacterial invasion, and accumulation of neutrophilic leukocytes. Secondary intestinal lesions may develop as extensions from the primary lesion (usually in the cecum, appendix, or nearby portion of the ascending colon). The organisms may travel to the ileocecal valve and terminal ileum, producing a chronic infection. The sigmoid colon and rectum are favored sites for later lesions. An amebic inflammatory or granulomatous tumorlike mass (ameboma) may form on the intestinal wall, sometimes growing sufficiently large to block the lumen.
Factors that determine invasion of amebae include the following: the number of amebae ingested, the pathogenic capacity of the parasite strain, host factors such as gut motility and immune competence, and the presence of suitable enteric bacteria that enhance amebic growth. Correct and prompt identification of the Entamoeba species remains a critical problem. Trophozoites, especially with red blood cells in the cytoplasm, found in liquid or semiformed stools are pathognomonic.
Symptoms vary greatly depending on the site and intensity of lesions. Extreme abdominal tenderness, fulminating dysentery, dehydration, and incapacitation occur in serious disease. In less acute disease, onset of symptoms is usually gradual and often includes episodes of diarrhea, abdominal cramps, nausea and vomiting, and an urgent desire to defecate. More frequently, there will be weeks of cramps and general discomfort, loss of appetite, and weight loss, with general malaise. Symptoms may develop within 4 days of exposure, may occur up to a year later, or may never occur.
Extraintestinal infection is metastatic and rarely occurs by direct extension from the bowel. By far the most common form is amebic hepatitis or liver abscess (4% or more of clinical infections), which is assumed to be due to microemboli, including trophozoites carried through the portal circulation. It is assumed that hepatic microembolism with trophozoites is a common accompaniment of bowel lesions but that these diffuse focal lesions rarely progress. A true amebic abscess is progressive, nonsuppurative (unless secondarily infected), and destructive without compression and formation of a wall. The contents are necrotic and bacteriologically sterile, active amebae being confined to the walls. A characteristic “anchovy paste” is produced in the abscess and seen on surgical drainage. More than half of patients with amebic liver abscess give no history of intestinal infection, and rarely, amebic abscesses occur elsewhere (eg, lung, brain, spleen). Any organ or tissue in contact with active trophozoites may become a site of invasion and abscess. Hepatic abscess, usually showing as an elevation of the right dome of the diaphragm, can be observed by ultrasonography, computerized tomography, magnetic resonance imaging, or radioisotope scanning. Serologic tests in these cases are usually strongly positive.
Invasive or pathogenic E histolytica is now considered a species distinct from the more common lumen-dwelling nonpathogenic commensal species, Entamoeba dispar, with the name E histolytica reserved only for the pathogenic form. E dispar and the related Entamoeba moshkovskii are, based on isoenzyme, genetic, and PCR analyses, distinct species, even though they are microscopically identical. E histolytica must be distinguished not only from E dispar and E moshkovskii but also from four other ameba-like organisms that are also intestinal parasites of humans: (1) E coli, which is very common; (2) Dientamoeba fragilis (a flagellate), the only intestinal parasite other than E histolytica that has been suspected of causing diarrhea and dyspepsia but is not invasive; (3) Iodamoeba bütschlii; and (4) Endolimax nana. Considerable experience is required to distinguish E histolytica from other forms, but it is necessary to do so because misdiagnosis often leads to unnecessary treatment, overtreatment, or failure to treat.
Enzyme immunoassay (EIA) kits are available commercially for serodiagnosis of amebiasis when stools are often negative. EIA tests to detect amebic antigen in the stool are also sensitive and specific for E histolytica and can distinguish between pathogenic and nonpathogenic infections (Haque et al, 2003).
E histolytica occurs worldwide, mostly in developing countries where sanitation and hygiene are poor. Infections are transmitted via the fecal–oral route; cysts are usually ingested through contaminated water, vegetables, and food; flies have also been linked to transmission in areas of fecal pollution. Most infections are asymptomatic, with the asymptomatic cyst passers being a source of contamination for outbreaks where sewage leaks into the water supply or breakdown of sanitation occurs (as in mental, geriatric, or children’s institutions or prisons).
Cryptosporidium species, typically Cryptosporidium hominis, can infect the intestine in immunocompromised persons (eg, those with AIDS) and cause severe, intractable diarrhea. They have long been known as parasites of rodents, fowl, rhesus monkeys, cattle, and other herbivores and have probably been an unrecognized cause of self-limited, mild gastroenteritis and diarrhea in humans. Oocysts measuring 4–5 μm are passed in feces in enormous numbers and are immediately infectious. When oocysts in contaminated foods and water are ingested, sporozoites excyst and invade intestinal cells; the parasites multiply asexually within the apical portion of the intestinal cells, are released, and infect other intestinal cells to begin a new cycle. They also reproduce sexually, forming male microgamonts and female macrogamonts that fuse and develop into the oocysts.
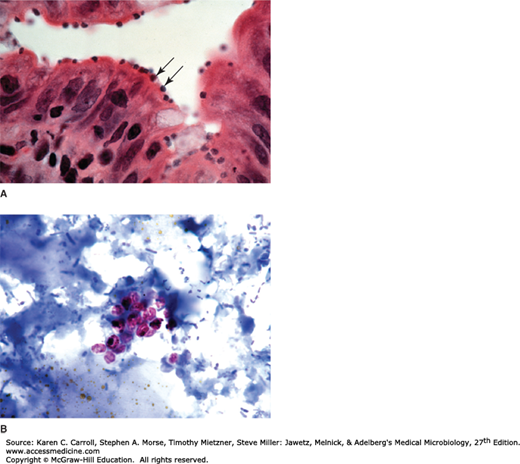
Cryptosporidium inhabits the brush border of mucosal epithelial cells of the gastrointestinal tract, especially the surface of villi of the lower small bowel (Figure 46-3A). The prominent clinical feature of cryptosporidiosis is watery diarrhea, which is mild and self-limited (1–2 weeks) in normal persons but may be severe and prolonged in immunocompromised or very young or old individuals. The small intestine is the most commonly infected site, but Cryptosporidium infections have also been found in other organs, including other digestive tract organs and the lungs.
FIGURE 46-3
Cryptosporidium. A: Histologic section of intestine with organisms (arrows) at the apical portion of the epithelial cells. (Courtesy of Pathology, UCSF.) B: Oocysts (4–5 μm) stain pink in stool samples stained with an acid-fast stain. (Used with permission from Sullivan J: A Color Atlas of Parasitology, 8th ed. 2009.)
Diagnosis depends on detection of oocysts in fresh stool samples. Stool concentration techniques using a modified acid-fast stain are usually necessary (Figure 46-3B), and monoclonal antibody–based tests are available that can detect low levels of fecal antigen.
The incubation period for cryptosporidiosis is from 1 to 12 days, and the disease is acquired from infected animal or human feces or from fecally contaminated food or water. For those at high risk (immunocompromised and very young or old persons), avoidance of animal feces and careful attention to sanitation are required. The organisms are widespread and probably infect asymptomatically a significant proportion of the human population. Occasional outbreaks, such as the one that occurred in Milwaukee in early 1993, affecting more than 400,000 people, can result from inadequate protection, treatment, or filtration of water supplies for large urban centers. In this instance, cattle manure from large dairy farms was the source of contamination of the water supply. As few as 30 organisms can initiate an infection—and the ability of the parasite to complete its life cycle, including the sexual phase, within the same individual makes possible the fulminating infections frequently observed in immunosuppressed individuals.
The life cycle of Cyclospora is similar to that of Cryptosporidium and appears to involve only a single host. Cyclospora, however, differs from Cryptosporidium in that Cyclospora oocysts are not immediately infectious when passed in stools. Unlike Cryptosporidium oocysts, which are infectious in the feces, Cyclospora oocysts take days or weeks to become infectious, and because of this, direct person-to-person transmission through fecal exposure is unlikely to occur. Cyclosporiasis has been linked to waterborne and foodborne infections from various types of fresh produce, including raspberries, mesclun, and basil, since the 1990s (Ortega and Sanchez, 2010).
Altered mucosal architecture with shortening of intestinal villi due to diffuse edema and infiltration of inflammatory cells leads to diarrhea, anorexia, fatigue, and weight loss. The duration of symptoms among untreated, nonimmune persons is often prolonged but ultimately self-limited, with remitting-relapsing symptoms lasting up to several weeks or months. The incubation period for Cyclospora infections is about 1 week, similar to infections with Cryptosporidium. Specific requests for laboratory testing of Cyclospora are necessary (same for Cryptosporidium) when examining stools for oocysts (8–10 μm), which are acid-fast positive (reddish). Unlike infections with Cryptosporidium, Cyclospora infections are treatable with trimethoprim–sulfamethoxazole (TMP-SMZ).
SEXUALLY TRANSMITTED PROTOZOAN INFECTION
Trichomonas vaginalis exists only as a trophozoite (no cyst stage); it has four free flagella that arise from a single stalk and a fifth flagellum, which forms an undulating membrane. It is pyriform and approximately 20 μm in length and 10 μm wide.
T vaginalis is sexually transmitted, and most infections are asymptomatic or mild for both women and men. In women, the infection is normally limited to the vulva, vagina, and cervix; it does not usually extend to the uterus. The mucosal surfaces may be tender, inflamed, eroded, and covered with a frothy yellow or cream-colored discharge. In men, the prostate, seminal vesicles, and urethra may be infected. Signs and symptoms in females, in addition to profuse vaginal discharge, include local tenderness, vulval pruritus, and burning. About 10% of infected males have a thin, white urethral discharge. The incubation period is from around 5 to 28 days.
T vaginalis is a common parasite of both males and females but infection is more common in women than in men. Infants may be infected during birth. In the United States, it is estimated that 3.7 million people have the infection but only 30% become symptomatic. Control of T vaginalis infections always requires simultaneous treatment of both sexual partners. Mechanical protection (condoms) should be used during intercourse until the infection is eradicated in both partners.
BLOOD AND TISSUE PROTOZOAN INFECTIONS
The hemoflagellates of humans include the genera Trypanosoma and Leishmania (Table 46-4). There are two distinct types of human trypanosomes: (1) African, which causes sleeping sickness and is transmitted by tsetse flies (eg, Glossina): Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense; and (2) American, which causes Chagas disease and is transmitted by kissing bugs (eg, Triatoma): Trypanosoma cruzi. The genus Leishmania, divided into a number of species infecting humans, causes cutaneous (Oriental sore), mucocutaneous (espundia), and visceral (kala-azar) leishmaniasis. All of these infections are transmitted by sandflies (Phlebotomus in the Old World and Lutzomyia in the New World).
| Hemoflagellates | Disease | Vector | Stages in Humans |
|---|---|---|---|
| Trypanosoma brucei rhodesiense | African sleeping sickness (acute) | Tsetse fly | Trypomastigotes in blood |
| Trypanosoma brucei gambiense | African sleeping sickness (chronic) | Tsetse fly | Trypomastigotes in blood |
| Trypanosoma cruzi | Chagas disease | Kissing bug | Trypomastigotes in blood; amastigotes intracellular |
| Leishmania spp. | Cutaneous, mucocutaneous, visceral leishmaniasis | Sandfly | Amastigotes intracellular in macrophages and monocytes |
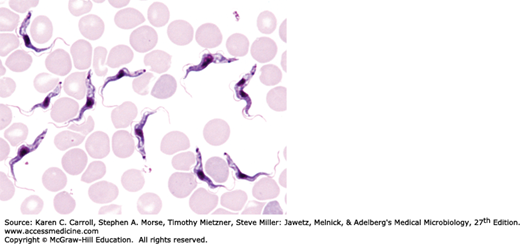
The genus Trypanosoma appears in the blood as trypomastigotes, with elongated bodies supporting a longitudinal lateral undulating membrane and a flagellum that borders the free edge of the membrane and emerges at the anterior end as a whiplike extension (Figure 46-4). The kinetoplast (circular DNA inside the single mitochondrion) is a darkly staining body lying immediately adjacent to the basal body from which the flagellum arises. T brucei rhodesiense, T brucei gambiense, and Trypanosoma brucei brucei (which causes a sleeping sickness called nagana in livestock and game animals) are indistinguishable morphologically but differ biochemically, ecologically, and epidemiologically.
Infective trypanosomes of T brucei gambiense and T brucei rhodesiense are introduced through the bite of the tsetse fly and multiply at the site of inoculation to cause variable induration and swelling (the primary lesion), which may progress to form a trypanosomal chancre. The African forms multiply extracellularly as trypomastigotes in the blood as well as in lymphoid tissues. They spread to lymph nodes, to the bloodstream, and, in terminal stages, to the central nervous system (CNS), where they produce the typical sleeping sickness syndrome: lassitude, inability to eat, tissue wasting, unconsciousness, and death.
CNS involvement is most characteristic of African trypanosomiasis. T brucei rhodesiense appears in the cerebrospinal fluid in about 1 month and T brucei gambiense in several months, but both are present in small numbers. T brucei gambiense infection is chronic and leads to progressive diffuse meningoencephalitis, with death from the sleeping syndrome usually following in 1–2 years. The more rapidly fatal T brucei rhodesiense produces somnolence and coma only during the final weeks of a terminal infection. The trypanosomes are transmissible through the placenta, and congenital infections occur in hyperendemic areas.
The African trypanosomes of the T brucei complex are remarkable in that they undergo antigenic variation through a series of genetically controlled surface glycoproteins that coat the surface of the organism (variant surface glycoproteins, or VSGs). Successive waves of parasites in the host bloodstream are each covered with a distinct coat. This process is due to genetically induced changes of the surface glycoprotein. By producing different antigenic surface membranes, the parasite is able to evade the host’s antibody response. Each population is reduced but is promptly replaced with another antigenic type before the preceding one is eliminated. Each trypanosome is thought to possess about 1000 VSG genes, an example of mosaic gene formation.
African trypanosomiasis is restricted to recognized tsetse fly belts. T brucei gambiense, transmitted by the streamside tsetse Glossina palpalis and several other humid forest tsetse vectors, extends from West to Central Africa and produces a relatively chronic infection with progressive CNS involvement. T brueci rhodesiense, transmitted by the woodland-savanna Glossina morsitans, Glossina pallidipes, and Glossina fuscipes, occurs in the eastern and southeastern savannas of Africa, with foci west of Lake Victoria. It causes a smaller number of cases but is more virulent. Bushbuck and other antelopes may serve as reservoirs of T brucei rhodesiense, whereas humans are the principal reservoir of T brucei gambiense. Control depends on searching for and then isolating and treating patients with the disease; controlling movement of people in and out of fly belts; using insecticides in vehicles; and instituting fly control, principally with aerial insecticides and by altering habitats. Contact with reservoir animals is difficult to control, and insect repellent is of little value against tsetse bites.
T cruzi has three developmental stages: epimastigotes in the vector, trypomastigotes (in the bloodstream), and a rounded intracellular stage, the amastigote. The blood forms of T cruzi are present during the early acute stage and at intervals thereafter in smaller numbers. They are typical trypomastigotes with a large, rounded terminal kinetoplast in stained preparations, but they are difficult to morphologically distinguish from African trypanosomes. The tissue forms, which are most common in heart muscle, liver, and brain, develop as amastigotes that multiply to form an intracellular colony after invasion of the host cell or phagocytosis of the parasite (Figure 46-5).
FIGURE 46-5
Trypanosoma cruzi amastigote colonies (arrows) in heart muscle. Amastigotes are 1–3 μm in diameter in tissue sections. (Used with permission from Sullivan J: A Color Atlas of Parasitology, 8th ed. 2009.) Diagram of an amastigote with the characteristic “dot” (nucleus) and “dash” (kinetoplast).
Infective forms of T cruzi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree