Pulmonary Resection
David J. Sugarbaker
Marcelo C. DaSilva
History
The evolution of thoracic surgery parallels the development of endotracheal anesthesia and selective lung ventilation. These techniques permit safe operations to be performed in an open thoracic cavity. Doctor Evarts Graham performed the first successful resection for lung cancer, a pneumonectomy, in St. Louis in 1933. Pulmonary surgery has grown in scope and complexity from the simple management of pleural infection in the early 1900s to parenchymal resection for the treatment of tuberculosis in the 1940s, to anatomical resection and video-assisted thoracic surgery (VATS) for the extirpation of lung cancer in the present era. The current accepted standard resection for a localized non–small cell lung cancer (NSCLC) is to remove the involved lobe of the lung along with its draining peribronchial and hilar lymph nodes.
Epidemiology
The overall incidence rates of cancer have decreased in both men and women, largely the result of declining rates for three major cancer sites: lung, prostate and colon, and rectum. In 2010, there were approximately 222,520 new cases of lung cancer (non–small cell and small cell combined) and 157,300 patient deaths in the United States, and lung cancer continues to be the leading cause of cancer-related mortality in this country. The 5-year relative survival rate for patients with lung cancer between 1999 and 2006 was 15.8%. The 5-year relative survival rate varies markedly depending on the stage at diagnosis, from 49% to 16% to 2% for patients with local, regional, and distant stage disease, respectively. In patients considered at high risk for developing lung cancer, no screening modality for early detection has been shown to alter mortality. Studies of newer screening technologies, such as low-dose computed tomography (CT) scans and biomarker screenings, report primarily on lung cancer detection rates and do not present sufficient data to determine whether exposure to these newer technologies will benefit or harm people.
Lung cancer is divided into two major histologic groups: small cell lung cancer (SCLC) and NSCLC. SCLC is more aggressive than NSCLC. Therefore, its treatment remains mostly nonsurgical. Left untreated, SCLC has a median survival of 2 to 4 months. Nevertheless, SCLC is responsive to systemic chemotherapy with median survival
approaching 18 to 36 months. Currently, SCLC accounts for 20% of new lung cancer cases per year in the United States. The other histologic group, NSCLC, is of three major subtypes: squamous cell carcinoma, adenocarcinoma including bronchioalveolar carcinoma (BAC), and large cell carcinoma. These tumors account for 80% of all lung malignancies. The single most important risk factor for the development of lung cancer is smoking. The risk for lung cancer is tenfold higher for smokers than for lifetime nonsmokers (defined as a person who has smoked <100 cigarettes in their lifetime). The risk increases with the quantity of cigarettes, duration of smoking, and starting age. Smoking cessation results in a decrease in precancerous lesions and a reduction in the risk of developing lung cancer. Asbestos exposure and cigarette smoking may exert a synergistic effect on the lung cancer risk. Smoking-related lung carcinogenesis is a multistep process. Squamous carcinoma and adenocarcinoma have defined premalignant precursor lesions. The lung epithelium undergoes morphological changes from hyperplasia, metaplasia, dysplasia, to carcinoma in situ. Dysplasia and carcinoma in situ are considered the principal premalignant lesions because they are more likely to progress to invasive cancer and less likely to spontaneously regress. In addition, the risk of developing a second lung cancer after resection of a NSCLC is approximately 1% to 2% per patient per year. Of those patients who develop a second NSCLC, approximately 50% can have these tumors resected. The median survival from the diagnosis of a second lung cancer is 1 to 2 years. The 5-year survival rate is approximately 20% (range, 4% to 32%). In patients who survive SCLC, the average risk of developing a second SCLC is approximately 6% per patient per year. It is important to note that survivors who continue to smoke cigarettes have an increased risk of developing a second lung cancer.
approaching 18 to 36 months. Currently, SCLC accounts for 20% of new lung cancer cases per year in the United States. The other histologic group, NSCLC, is of three major subtypes: squamous cell carcinoma, adenocarcinoma including bronchioalveolar carcinoma (BAC), and large cell carcinoma. These tumors account for 80% of all lung malignancies. The single most important risk factor for the development of lung cancer is smoking. The risk for lung cancer is tenfold higher for smokers than for lifetime nonsmokers (defined as a person who has smoked <100 cigarettes in their lifetime). The risk increases with the quantity of cigarettes, duration of smoking, and starting age. Smoking cessation results in a decrease in precancerous lesions and a reduction in the risk of developing lung cancer. Asbestos exposure and cigarette smoking may exert a synergistic effect on the lung cancer risk. Smoking-related lung carcinogenesis is a multistep process. Squamous carcinoma and adenocarcinoma have defined premalignant precursor lesions. The lung epithelium undergoes morphological changes from hyperplasia, metaplasia, dysplasia, to carcinoma in situ. Dysplasia and carcinoma in situ are considered the principal premalignant lesions because they are more likely to progress to invasive cancer and less likely to spontaneously regress. In addition, the risk of developing a second lung cancer after resection of a NSCLC is approximately 1% to 2% per patient per year. Of those patients who develop a second NSCLC, approximately 50% can have these tumors resected. The median survival from the diagnosis of a second lung cancer is 1 to 2 years. The 5-year survival rate is approximately 20% (range, 4% to 32%). In patients who survive SCLC, the average risk of developing a second SCLC is approximately 6% per patient per year. It is important to note that survivors who continue to smoke cigarettes have an increased risk of developing a second lung cancer.
Patients with resectable disease may be cured by surgery or surgery with adjuvant chemotherapy. Local control can be achieved with radiation therapy in a large number of patients with unresectable disease, but cure is seen only in a small number of patients. Patients with locally advanced, unresectable disease may have long-term survival with radiation therapy combined with chemotherapy. Patients with advanced metastatic disease may achieve improved survival and palliation of symptoms with chemotherapy.
Surgery is the treatment of choice for early-stage NSCLC (stages I and II). For stage III and IV NSCLC the treatment of choice is generally palliative. Patients are usually treated under a multimodality protocol, although selected patients with stage III disease have the potential for cure with resection depending on the degree of invasion of local structures and the extent of mediastinal lymph node involvement. Neoadjuvant strategies involving chemotherapy, thoracic radiation, or both, can render some of these patients subsequently resectable. In patients thought to have resectable lesions without mediastinal nodal involvement, surgery remains the best curative option.
Nsclc Staging System
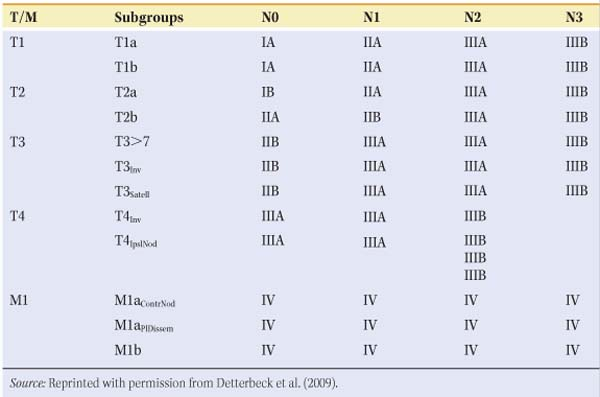
The seventh edition of the lung cancer stage classification system was adopted in the United States on January 1, 2010. Having an accurate and reproducible staging system is critical for defining prognostic subgroups, planning treatment strategies, and analyzing the results of clinical trials. The Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) are the organizations that periodically review, refine, and define the stage classification systems. The TNM stage classification system was developed to enable classification of tumor extension (TX to 4), nodal involvement (NX to 3), and distant metastatic spread (M0 to 1a,b). The most recent (seventh) International Lung Cancer Staging system is shown in Table 1. The two most common types of stage assessment are clinical staging (determined by using all the information available pretreatment) and pathologic staging (determined after resection). Clinical stage is denoted by the prefix “c” and pathologic stage by the prefix “p.” Major differences in the recent edition include the deletion of category MX (i.e., cannot be assessed or proved), because clinical staging is always available. While the T descriptor definitions maintain a size threshold of 3 cm as the cutpoint between a T1 and T2 tumor, several new cutpoints have been identified at 2, 5, and 7 cm. Tumors >7 cm in size are classified as T3, since they have a survival rate similar to other T3 tumors characterized by invasion or central location. No survival differences were shown between cT1a, cT1b, and cT2a, although this probably was the result of patient numbers too small to yield statistically significant values. Another modification of the seventh edition is related to additional tumor nodules. Patients with additional satellite nodules in the same lobe as the primary tumor have survival rates similar to T3 and now are classified as T3satell, where previously they were classified as T4. Survival with T3satell is statistically significantly better than survival with T4. Patients with an ipsilateral nodule in a different lobe are classified as T4IpisNod or simply T4, whereas they used to be classified as M1. Patients with pleural dissemination are now classified as M1a, as their prognosis is statistically significantly worse than T4. The N descriptor categorization (i.e., N0, N1, N2, and N3) has not changed. No survival differences were found between patients with involvement of only peripheral (N1) nodes versus hilar (N2) nodes, and there were no survival differences based on which N2 nodal stations were involved. In examining the effect of skip metastases (involvement of an N2 station without any N1 stations), survival in patients with right upper lobe tumors and N2-positive nodes, with or without N1 nodes, was not altered, although there was a small difference among such patients when the tumor was in the left upper lobe. The number of involved nodal zones does appear to have prognostic impact. Patients with single-zone N1 or N2 involvement have better survival than those with multizone N1 and N2 involvement (5-year survival rate of 48% vs. 35%, P < 0.09, and 34% vs. 20%, P < 0.001, respectively). Finally, after the above modifications, the cohorts remaining in the M descriptor separated into two distinct prognostic groups. M1a is reserved for patients with either pleural dissemination or contralateral pulmonary nodules. The M1b subgroup is reserved for distant metastases.
The revised TNM associations with stage groupings are shown in Table 2. For stages I through IIB, surgical therapy is generally offered as monotherapy, at times followed by chemotherapy. Stage IIIA (positive ipsilateral tracheal or subcarinal nodes) is best treated preoperatively with chemotherapy or chemoradiation followed by resection in responders. Several small, randomized trials suggest a doubling of the 5-year survival rate with this strategy. Patients with residual disease have a very poor 5-year survival prognosis. Resection of tumors with contralateral paratracheal positive nodes (IIIB) is controversial with a worse 5-year survival prognosis than stage IIIA. Surgical resection for stage IV patients is generally not indicated except in the few patients who may have isolated and treatable brain or adrenal metastases. Revisions in the system for classifying regional lymph node stations for lung cancer staging were also adopted in the seventh edition of the TNM classification for lung cancer.
Table 1 T, N, M Descriptors for the International Lung Cancer Staging System (7th Edition) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
At the time of diagnosis, patients with NSCLC can be divided into three treatment groups. The first group comprises patients with surgically resectable disease (stage I, stage II, and selected stage III). Patients in this group have the best prognosis. Patients with resectable disease who have medical contraindications to surgery are candidates for curative radiation therapy. Adjuvant cisplatin-based combination chemotherapy may provide a survival advantage to patients with resected stage II or stage IIIA NSCLC. The second group includes patients with either locally or regionally advanced disease (T3 and T4) and/or (N2 and N3). This group has a diverse natural history. Selected patients with locally advanced tumors may benefit from combined modality treatments. Patients with unresectable or N2 and N3 disease are treated with radiation therapy in combination with chemotherapy. Selected patients with T3 or N2 disease can be treated effectively with surgical resection and either preoperative or postoperative chemotherapy or chemoradiation therapy. The third group includes patients with distant metastatic disease (M1) that was found at the time of diagnosis. This group can be treated with radiation therapy or chemotherapy for palliation of symptoms from the primary tumor. Patients with good performance status (PS), women, Patients with good performance status (PS), women, and patients with a distant solitary metastasis have better survival than patients with multiple distant metastases (median survival time, 5 vs 6 months, respectively; 1-year survival rate, 20% vs 23%, respectively; p = 0.006). Platinum-based chemotherapy has been associated with short-term palliation of symptoms and with a survival advantage. Patients previously treated with platinum combination chemotherapy may derive symptom control and survival benefit from docetaxel, pemetrexed, or epidermal growth factor receptor inhibitor. Currently, no single chemotherapy regimen can be recommended for routine use. Therefore, surgery remains the cornerstone for curative therapy for NSCLC.
Noninvasive Staging
A standard posteroanterior and lateral chest radiograph can reveal discrete mass lesions as well as atelectasis and postobstructive pneumonia due to the presence of endobronchial tumors. Hilar and mediastinal adenopathy, along with pleural effusion, might also be noted. Traditionally, assessment of mediastinal lymph nodes in lung cancer patients is performed with a chest CT scan, which should include examination of the liver and adrenal glands along with mediastinal lymph node stations. Overall accuracy of CT in mediastinal staging for NSCLC is only 0.75 to 0.80, with 20% to 40% false-negative and 18% to 23% false-positive results. A lymph node is considered malignant when its shortest axis is at least 10 mm. The point of concern is that CT only depicts the shape and size of the mediastinal lymph nodes rather than usual tumor involvement.
The four most common sites of NSCLC metastases (stage IV disease) are the brain, bone, liver, and adrenal glands. Therefore, head CT scans, brain magnetic resonance imaging (MRI), and bone scans are often part of the metastatic evaluation to rule out central nervous system and bone involvement. In the absence of central nervous or bony symptoms in patients presenting with small tumors, the incidence of a true positive finding will be less than 10%. If these sites are excluded, the disease is confined to the chest (stages I to IIIB). The presence of pleural effusion, unresectable T4 tumors, and contralateral nodal involvement can be suggested by chest CT scan.
Positron emission tomography (PET) relies on the uptake and concentration of
2,3-fluorodeoxyglucose (FDG) in lung cancer cells. It is more sensitive and specific (approximately 90% sensitive and specific) for mediastinal nodal metastases than CT. PET scan is also fairly sensitive in detecting metastatic disease throughout the body outside the brain. PET has an overall sensitivity of 96% (range, 83% to 100%), specificity of 79% (range, 52% to 100%), and accuracy of 91% (range, 86% to 100%).10 False-negative results can occur in lesions smaller than 1 cm because a critical mass of metabolically active malignant cells is required for PET diagnosis. Lowe et al. found a sensitivity of 80% in lesions smaller than 1.5 cm compared with 92% in larger lesions. False negatives may also occur in tumors with a low metabolism, like carcinoid tumors and bronchioloalveolar cell carcinomas. False-positive FDG uptake is seen in inflammatory conditions such as bacterial pneumonia; pyogenic abscesses; aspergillosis; and granulomatous diseases such as tuberculosis, sarcoidosis, histoplasmosis, Wegener’s granulomatosis, and coal miner’s lung. In these lesions the FDG uptake has been attributed to granulocyte and/or macrophage activity.
2,3-fluorodeoxyglucose (FDG) in lung cancer cells. It is more sensitive and specific (approximately 90% sensitive and specific) for mediastinal nodal metastases than CT. PET scan is also fairly sensitive in detecting metastatic disease throughout the body outside the brain. PET has an overall sensitivity of 96% (range, 83% to 100%), specificity of 79% (range, 52% to 100%), and accuracy of 91% (range, 86% to 100%).10 False-negative results can occur in lesions smaller than 1 cm because a critical mass of metabolically active malignant cells is required for PET diagnosis. Lowe et al. found a sensitivity of 80% in lesions smaller than 1.5 cm compared with 92% in larger lesions. False negatives may also occur in tumors with a low metabolism, like carcinoid tumors and bronchioloalveolar cell carcinomas. False-positive FDG uptake is seen in inflammatory conditions such as bacterial pneumonia; pyogenic abscesses; aspergillosis; and granulomatous diseases such as tuberculosis, sarcoidosis, histoplasmosis, Wegener’s granulomatosis, and coal miner’s lung. In these lesions the FDG uptake has been attributed to granulocyte and/or macrophage activity.
Table 2 Stage Groups According to TNM Descriptor and Subgroups | |
|---|---|
|
Transesophageal endoscopic ultrasound scanning (EUS) is a new minimally invasive method that provides high-resolution imaging of the mediastinum by using high-frequency ultrasound probes attached to the tip of a flexible endoscope. It offers the additional facility of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) or tru-cut biopsy (TCB) under real-time ultrasound guidance.
EUS-FNA permits access to the posterior mediastinum and tissue acquisition under real-time ultrasound guidance through the esophageal wall. It accesses a largely nonoverlapping area when compared with mediastinoscopy. EUS-FNA can be used to assess mediastinal lymph nodes at most levels, particularly at levels 4 left, 5, 7, 8, and 9 as well as metastasis to the left adrenal gland. Characteristics of lymph nodes indicative of malignancy are hypoechoic core, sharp edges, round shape, and long axis diameter >10 mm. Indications for EUS-FNA in the mediastinum include the need to obtain tissue for diagnosis of a primary lesion or to sample tissue from mediastinal lymph nodes for lung cancer staging. EUS-FNA can be done on an outpatient basis, is well tolerated, and provides an excellent diagnostic yield with a sensitivity of more than 90% and a specificity of 100%. Compared with CT, PET, mediastinoscopy, or transbronchial aspiration, it is significantly more accurate for staging NSCLC. However, mediastinoscopy remains the gold standard for evaluating the anterior mediastinum, since the air-filled trachea precludes imaging by EUS in this region.
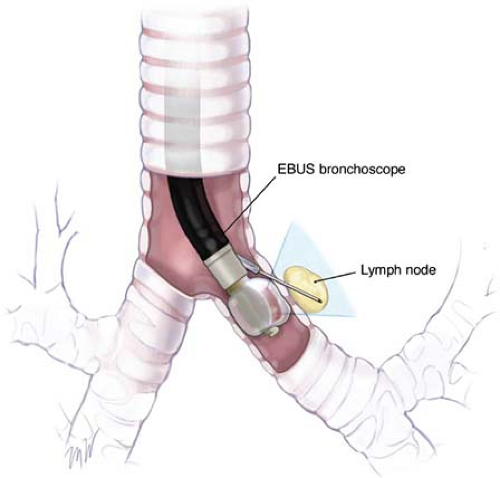
Recently, endobronchial ultrasound-guided transbronchial needle aspiration biopsy (EBUS-TBNA) has been developed (Fig. 1). It has sensitivity of more than 90% in the staging of NSCLC. When used in combination for mediastinal staging, EUS-FNA and EBUS-TBNA have a documented sensitivity and specificity of 100%. It therefore seems logical to assume that the combination of EUS-FNA and EBUS-TBNA will replace more invasive diagnostic methods in the staging of lung cancers.
Surgical Staging
Treatments for lung cancer are largely decided on the basis of (a) accurate identification of histological type (small cell vs. NSCLC), (b) mediastinal lymph node involvement, and (c) distant metastasis. Involvement of the mediastinal lymph nodes is suggested by the finding of nodes that are larger than 10 mm in cross-sectional diameter on CT scan. The accuracy of CT scanning in identifying tumor-bearing mediastinal lymph nodes is 60% to 70%. Because of the high false-positive rate with chest CT scanning, we do not consider a patient inoperable before performing a cervical mediastinoscopy (CM), EUS-FNA, and/or EBUS-TBNA to sample the mediastinal lymph nodes for histologic examination.
Nearly half of patients with lung cancer have mediastinal disease at diagnosis. Patients with positive N2 lymph nodes have a very different prognosis compared with patients with negative N2 lymph nodes. Metastases to ipsilateral or subcarinal lymph nodes (N2) are classified as stage IIIA disease. Many centers treat the latter group of patients with multimodality therapy including radiation and chemotherapy, with surgery reserved for treatment under investigational protocols. Direct mediastinal invasion (T4) or metastasis to contralateral mediastinal lymph nodes (N3) is classified as stage IIIB disease. The 5-year survival rate in this subgroup is <5%. Patients are generally offered treatment with chemotherapy alone.
Bronchoscopy
Before thoracotomy is performed, the surgeon should perform flexible fiberoptic bronchoscopy to evaluate for anatomic abnormalities, endobronchial tumor extent, and previously undetected lesions.
Cervical Mediastinoscopy
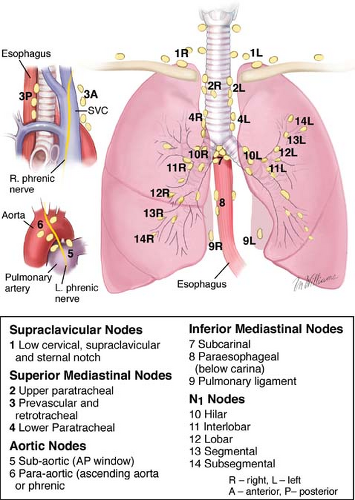
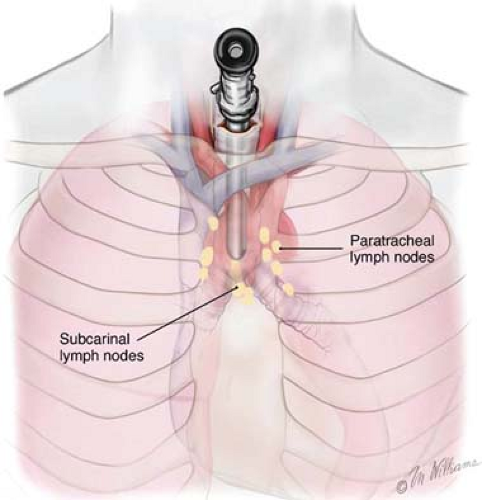
The intrathoracic lymph nodes are divided into anatomic stations (Fig. 2). CM can assess lymph node levels 2 left and right (upper paratracheal), 4 left and right (lower paratracheal), and 7 (subcarinal). Sensitivity for CM may vary from 0.44 to 0.92, and specificities and positive predictive values of 1.00 may be expected. Extended cervical mediastinoscopy (ECM) reaches lymph node levels 5 and 6. Despite its sensitivity, accuracy, and negative predictive values of 0.69, 0.91, and 0.89, respectively, ECM has not been widely accepted in clinical practice.
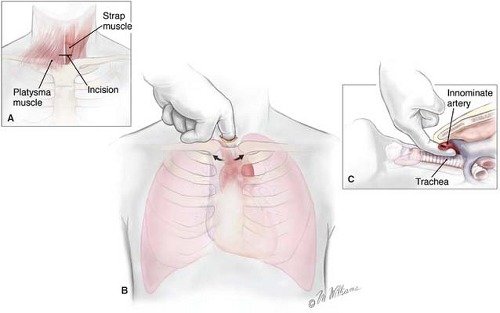
CM is performed by positioning the patient supine with the neck fully extended. A small transverse incision is made one fingerbreadth above the sternal notch, and the tissue planes are dissected between the strap muscles down to the pretracheal fascia (Fig. 3A to C). This plane is entered, and finger dissection is used to develop a plane along the anterior surface of the trachea posterior to the innominate artery down to the aortic arch (Fig. 4). Nodes cranial to the innominate vessels are termed level 2 nodes. Nodes caudad to the innominate artery are termed level 4 nodes. Subcarinal nodes are level 7. On the left, care is taken to identify and preserve the left recurrent laryngeal nerve as it courses medially to reach the tracheoesophageal groove. Cautery is generally avoided on the left side of the trachea to avoid injury to the nerve. Further dissection along each mainstem bronchus permits the surgeon to sample the tracheobronchial angle nodes bilaterally. CM is a safe procedure with mortality rates <0.1% and complication rates <1%, including paresis of the left laryngeal recurrent nerve, pneumothorax, pneumonia, injury to the azygos vein, perforation of the esophagus, mediastinitis, and hemorrhage. Nevertheless, bleeding is usually minor and can be controlled with packing. Major bleeding can be controlled with packing only, while one is getting ready to open the chest. Bleeding from major vessels, e.g., superior vena
cava, azygos vein, brachiocephalic artery, aortic arch, and pulmonary artery, will require expeditious median sternotomy. Small pneumothoraces can occasionally be managed conservatively. A chest X-ray with a new pleural effusion indicates blood draining into the pleural space and one should not hesitate to place a chest tube for drainage. Tracheobronchial injuries can result in a dramatic subcutaneous emphysema or air leak. Most of these injuries are small and will require observation only, but large injuries will require primary repair. Small, contained esophageal leaks can be managed conservatively. Large injuries will require complex repair and management, which is beyond the scope of this chapter.
cava, azygos vein, brachiocephalic artery, aortic arch, and pulmonary artery, will require expeditious median sternotomy. Small pneumothoraces can occasionally be managed conservatively. A chest X-ray with a new pleural effusion indicates blood draining into the pleural space and one should not hesitate to place a chest tube for drainage. Tracheobronchial injuries can result in a dramatic subcutaneous emphysema or air leak. Most of these injuries are small and will require observation only, but large injuries will require primary repair. Small, contained esophageal leaks can be managed conservatively. Large injuries will require complex repair and management, which is beyond the scope of this chapter.
The information gained from mediastinal lymph node biopsy permits accurate pathologic staging of the disease before the decision for definitive resection is made. The presence of nodal spread to the ipsilateral mediastinal stations or subcarinal area confirms the diagnosis of stage IIIA disease, and surgery is offered only after neoadjuvant therapy, usually involving a platinum-based regimen of chemotherapy with or without radiation therapy. In instances in which the disease has spread to the contralateral mediastinal lymph nodes or to the supraclavicular nodes on either side (stage IIIB), curative resection is not possible, and surgery is offered only in a multimodality protocol setting.
Anterior Mediastinoscopy
Anterior mediastinoscopy is indicated for lesions in the anterior and superior mediastinum or hila, when CM is contraindicated. For tumors originating in the left
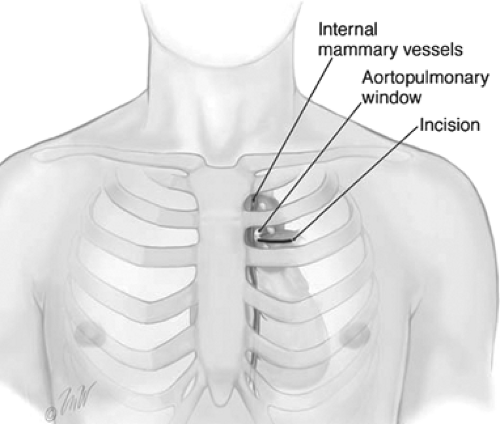
upper lobe, the stations of mediastinal lymph nodes most frequently involved are the periaortic arch (level 6) and aortopulmonary (AP) window (level 5) nodes. These nodes are difficult to access through a standard cervical approach. To perform anterior mediastinoscopy, the patient is placed in the supine position. A small 2-cm incision is made in the second intercostal space on the left side (Fig. 5). The pectoral muscle fibers are split exposing the intercostal muscles, the pleura is identified, and the mediastinum is entered. An extrapleural dissection is carried down onto the aortic arch and AP window. The internal mammary vessels are identified and swept medially or can be ligated. The mediastinal pleura is swept away laterally and the mediastinoscope is introduced through this incision into the mediastinum. Level 5 and level 6 lymph nodes are sampled, and care is taken to avoid the use of electrocautery near the recurrent laryngeal nerve, which arises from the left vagus nerve and travels under the aortic arch medially to the ligamentum arteriosum.
upper lobe, the stations of mediastinal lymph nodes most frequently involved are the periaortic arch (level 6) and aortopulmonary (AP) window (level 5) nodes. These nodes are difficult to access through a standard cervical approach. To perform anterior mediastinoscopy, the patient is placed in the supine position. A small 2-cm incision is made in the second intercostal space on the left side (Fig. 5). The pectoral muscle fibers are split exposing the intercostal muscles, the pleura is identified, and the mediastinum is entered. An extrapleural dissection is carried down onto the aortic arch and AP window. The internal mammary vessels are identified and swept medially or can be ligated. The mediastinal pleura is swept away laterally and the mediastinoscope is introduced through this incision into the mediastinum. Level 5 and level 6 lymph nodes are sampled, and care is taken to avoid the use of electrocautery near the recurrent laryngeal nerve, which arises from the left vagus nerve and travels under the aortic arch medially to the ligamentum arteriosum.
Thoracoscopy
Although CM remains the procedure of choice, especially for the higher mediastinal levels (2, and 4), in some situations CM can be technically challenging or contraindicated. In cases of previous laryngectomy, large goiters, and previous mediastinoscopy in patients who have neoadjuvant chemotherapy, thoracoscopic nodal sampling can be performed. The subaortic nodes (level 5) and paraaortic nodes (level 6) cannot be reached by routine CM, but can be biopsied easily by left thoracoscopy, and thoracoscopic sampling is a useful alternative to anterior mediastinoscopy in staging left upper lobe tumors. The posterior subcarinal (level 7), paraesophageal (level 8), and inferior pulmonary ligament nodes (level 9) can be sampled by thoracoscopy. In addition to evaluating N2 disease, thoracoscopy is particularly useful in ruling out unresectable T4 invasion in primary lung cancer and T3 invasion in high-risk patients.
The thoracoscopic approach to the AP window places the camera port in the seventh intercostal space at the midaxillary line. The second and third ports are placed according to the line of the planned thoracotomy incision. The second port is in the anterior axillary line, and the third is placed below the tip of the scapula. The lung is retracted inferiorly, and then the mediastinal pleura is opened along the inferior margin of the aortic arch. Using grasping forceps, subaortic nodes are drawn back, and a hemoclip is applied to the base of the vascular pedicle before the nodal pedicle is amputated. To sample inferior pulmonary ligament nodes, the inferior pulmonary ligament is divided using electrocautery and the nodal basin sampled by clamping the nodes and resecting them, using electrocautery. Alternatively, a single port (uniport) thoracoscopic biopsy may be performed.
Estimation of Pulmonary Reserve
The extent of surgery necessary for complete resection is often uncertain before the operation. Therefore, an assessment should be made as to whether the patient might tolerate a pneumonectomy, lobectomy, or a limited resection (segmental or wedge resection). A thorough physical examination, past medical history, social history including tobacco smoking and exposure to arsenic and asbestos, medications, a PA and lateral chest X-ray, CT scan, PET–CT scan, MRI, and pulmonary function tests are part of the preoperative assessment (Fig. 6 algorithm).
The majority of patients are current or former smokers; coexistent emphysema and obstructive pulmonary insufficiency are frequent companions to the primary malignancy. For these reasons, a careful preoperative assessment of pulmonary reserve and cardiac function is mandatory. Cessation of smoking at least 2 weeks (and preferably 6 weeks) before resection aids in the perioperative control of secretions and the avoidance of pneumonia. Risk assessment is a complex process, and one should focus on determining the individual patient’s resectability and operability. Resectability refers to the amount of lung tissue and tumor that can be safely removed without developing respiratory insufficiency. It depends directly on the amount of pulmonary reserve of the patient. Operability refers to the ability of a patient to survive the proposed procedure and its perioperative complications. It depends mostly on the patient’s comorbid conditions. However, neither operability of an individual patient nor resectability of a tumor should influence the decision concerning the role of a complete resection on survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree