24
Pulmonary Pharmacology
This chapter will be most useful after having a basic understanding of the material in Chapter 36, Pulmonary Pharmacology in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition contains:
• A description of the pathogenesis of asthma, including Figure 36-1: Cellular mechanisms of asthma
• A description of the pathogenesis of chronic obstructive pulmonary disease (COPD), including Figure 36-2: Cellular mechanisms in chronic obstructive pulmonary disease
• A description of the various routes of drug delivery to the lungs, including Figure 36-3: Schematic representation of the deposition of inhaled drugs
• The molecular structures of some of the drugs used to treat asthma, COPD, and other pulmonary diseases
LEARNING OBJECTIVES
 Understand the mechanisms of action of bronchodilator drugs used to relax airway smooth muscle and drugs used to prevent bronchoconstriction.
Understand the mechanisms of action of bronchodilator drugs used to relax airway smooth muscle and drugs used to prevent bronchoconstriction.
 Understand the anti-inflammatory mechanism of action of corticosteroids, and the role of inhaled and oral corticosteroids in the pharmacotherapy of asthma.
Understand the anti-inflammatory mechanism of action of corticosteroids, and the role of inhaled and oral corticosteroids in the pharmacotherapy of asthma.
 Understand the mechanism of action of mucolytic agents.
Understand the mechanism of action of mucolytic agents.
 Understand the mechanisms of action of antitussive drugs.
Understand the mechanisms of action of antitussive drugs.
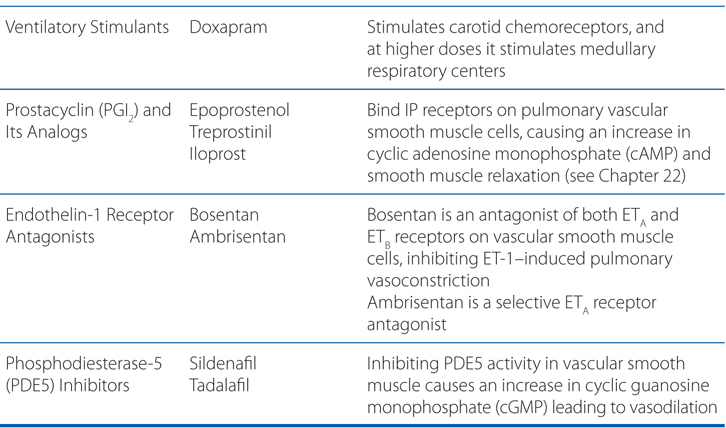
 Understand the mechanisms of action of drugs used to treat pulmonary artery hypertension (PAH).
Understand the mechanisms of action of drugs used to treat pulmonary artery hypertension (PAH).
 Know the untoward effects of the various bronchodilator drugs, corticosteroids, antitussive drugs, and drugs used to treat PAH.
Know the untoward effects of the various bronchodilator drugs, corticosteroids, antitussive drugs, and drugs used to treat PAH.
 Know which patients should be treated and when treatment should be initiated in patients with asthma, COPD, and PAH.
Know which patients should be treated and when treatment should be initiated in patients with asthma, COPD, and PAH.
 Know which drugs are most effective in treating patients with asthma, COPD, and PAH.
Know which drugs are most effective in treating patients with asthma, COPD, and PAH.
 Know which drugs can be used in combination to treat asthma, COPD, and PAH.
Know which drugs can be used in combination to treat asthma, COPD, and PAH.
DRUGS INCLUDED IN THIS CHAPTER
• Albuterol (salbutamol; VENTOLIN, PROVENTIL, ACCUNEB, others)
• Ambrisentan (LETAIRIS)
• Arformoterol (BROVENA)
• Beclomethasone dipropionate (QVAR)
• Benzonatate (TESSALON, others)
• Bosentan (TRACLEER)
• Budesonide (PULMICORT, others)
• Budesonide/formoterol (SYMBICORT)
• Ciclesonide (ALVESCO, OMNARIS)
• Codeine
• Dextromethorphan
• DNAase (dornase alfa, PULMOZYME)
• Doxapram (DOPRAM, others)
• Epoprostenol (prostacyclin, PGI2; FLOLAN, others)
• Flunisolide (AEROBID)
• Fluticasone (AEROSPAN, FLOVENT)
• Fluticasone/salmeterol (ADVAIR)
• Formoterol (FORADIL, others)
• Guaifenesin
• Hydrocortisone
• Iloprost (VENTAVIS)
• Indacaterol (ARCAPTA NEOHALER)
• Ipratropium bromide (ATROVENT, others)
• Ipratropium/albuterol (COMBIVENT, DUONEB, others)
• Levalbuterol (XOPENEX)
• Metaproterenol (ALUPENT, METAPREL)
• Methylprednisolone
• Mometasone (ASMANEX)
• Montelukast (SINGULAIR)
• N-acetylcysteine (MUCOMYST, others)
• Omalizumab
• Pirbuterol (MAXAIR)
• Prednisolone
• Prednisone
• Salmeterol (SEREVENT)
• Sildenafil (REVATIO)
• Tadalafil (ADCIRCA)
• Terbutaline (BRETHINE, others)
• Tiotropium bromide (SPIRIVA)
• Treprostinil (REMODULIN, TYVASO)
• Triamcinolone (AZMACORT, others)
• Zafirlukast (ACCOLATE)
• Zileuton (ZYFLO)
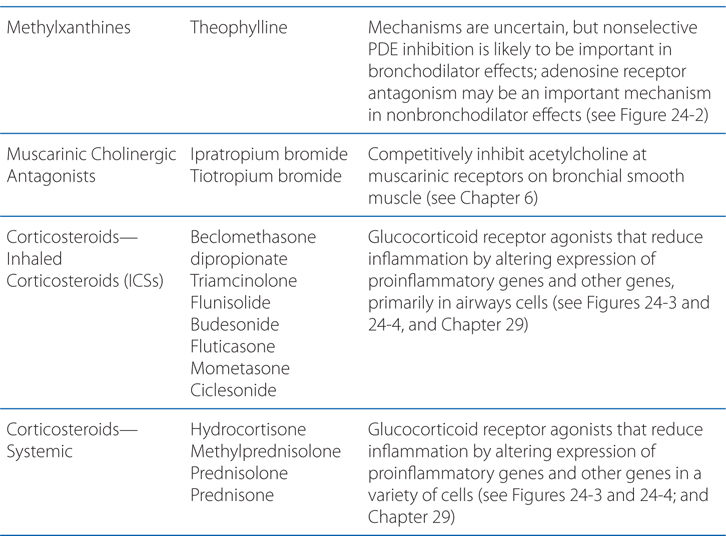
MECHANISMS OF ACTION OF DRUGS USED TO TREAT PULMONARY DISORDERS
INDIRECT ACTIONS OF β2 AGONISTS TO CAUSE BRONCHODILATION
• Prevention of mediator release from human lung mast cells (via β2 receptors)
• Prevention of microvascular leakage and thus the development of bronchial mucosal edema after exposure to mediators, such as histamine and leukotriene D4
• Increase in mucus secretion from submucosal glands and ion transport across airway epithelium; these effects may enhance mucociliary clearance, and thereby reverse the defective mucus clearance found in asthma
• Reduction in neurotransmission in human airway cholinergic nerves by an action at presynaptic β2 receptors to inhibit acetylcholine release, which may contribute to their bronchodilator effect by reducing reflex cholinergic bronchoconstriction
• Muscle tremor (direct effect on skeletal muscle β2 receptors)
• Tachycardia (direct effect on atrial β2 receptors, reflex effect from increased peripheral vasodilation via β2 receptors)
• Hypokalemia (direct β2 effect on skeletal muscle uptake of K+)
• Restlessness
• Hypoxemia (increased  mismatch due to reversal of hypoxic pulmonary vasoconstriction)
mismatch due to reversal of hypoxic pulmonary vasoconstriction)
• Local side effects
▶ Dysphonia
▶ Oropharyngeal candidiasis
▶ Cough
• Systemic side effects
▶ Adrenal suppression and insufficiency
▶ Growth suppression
▶ Bruising
▶ Osteoporosis
▶ Cataracts
▶ Glaucoma
▶ Metabolic abnormalities (glucose, insulin, triglycerides)
▶ Psychiatric disturbances (euphoria, depression)
▶ Pneumonia
An 8-year-old boy has occasional episodes of mild asthma while playing basketball with his friends.
a. What are the symptoms of asthma and some of the underlying pathogenic events that cause these symptoms?
Asthma is characterized by variable airflow obstruction caused by airway smooth muscle contraction. It is a chronic inflammatory disease of the airways that is characterized by activation of mast cells, infiltration of eosinophils and T helper 2 (TH2) lymphocytes. Mast cell activation by allergens and physical stimuli releases bronchoconstrictor mediators, such as histamine, leukotriene D4, and prostaglandin D2, which cause bronchoconstriction, microvascular leakage, and plasma exudation. Increased numbers of mast cells in airway smooth muscle are a characteristic of asthma.
The mechanism of chronic inflammation in asthma is still not well understood. It may initially be driven by allergen exposure, but it appears to become autonomous so that asthma is essentially incurable. Asthma usually starts in early childhood, and then may disappear during adolescence and reappear in adulthood. Asthma severity usually does not change so that patients with mild asthma rarely progress to severe asthma, and patients with severe asthma usually have this from the onset, although some patients, particularly with late-onset asthma, show a progressive loss of lung function like patients with COPD.
b. What treatment, if any, should this patient receive to relieve symptoms during an asthma attack?
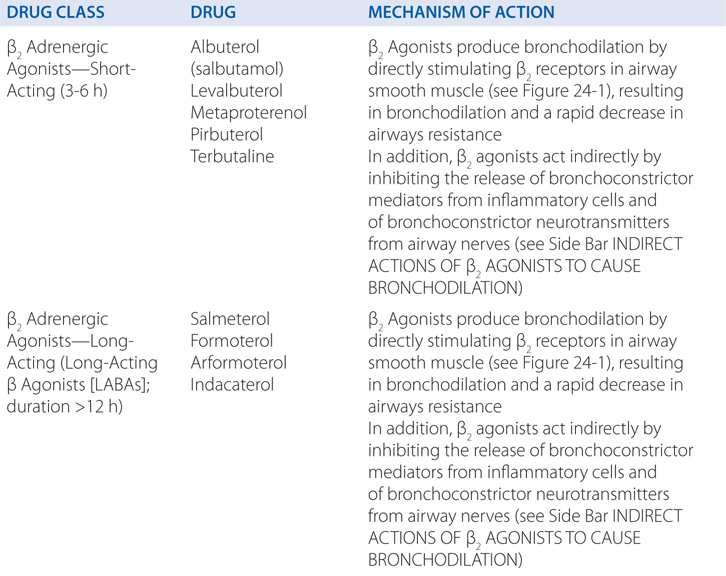
Inhaled short-acting β2 agonists are the most widely used and effective bronchodilators in the treatment of asthma due to their functional antagonism of bronchoconstriction. When inhaled from pressurized metered-dose inhalers (pMDIs) or dry powder inhalers (DPI), they are convenient, easy to use, rapid in onset, and without significant systemic side effects. In addition to their acute bronchodilator effect, these agents are effective in protecting against various challenges, such as exercise, cold air, and allergens.
c. What are the mechanisms of action of β2 agonists in treating asthma?
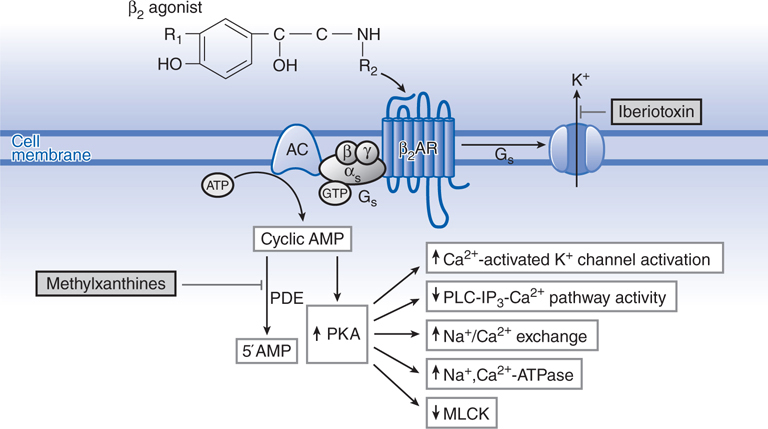
β2 Agonists produce bronchodilation by directly stimulating β2 receptors in airway smooth muscle (see Figure 24-1). β2 Agonists act as functional antagonists and reverse bronchoconstriction irrespective of the contractile agent. This is an important property for the treatment of asthma because many bronchoconstrictor mechanisms (neurotransmitters and mediators) are likely to be contributory. β2 Agonists may also indirectly cause bronchodilation by inhibiting the release of bronchoconstrictor mediators from inflammatory cells and of bronchoconstrictor neurotransmitters from airway nerves (see Side Bar INDIRECT ACTIONS OF β2 AGONISTS TO CAUSE BRONCHODILATION). Although these additional effects of β2 agonists may be relevant to the prophylactic use of these drugs against various challenges, their rapid bronchodilator action is probably attributable to a direct effect on airway smooth muscle.
FIGURE 24-1 Molecular actions of β2 agonists to induce relaxation of airway smooth muscle cells. Activation of β2 receptors (β2AR) results in activation of adenylyl cyclase (AC) via a stimulatory G protein intracellular cyclic adenosine monophosphate (cAMP) and activation of protein kinase A (PKA). PKA phosphorylates a variety of target substrates, resulting in opening of Ca2+-activated K+ channels (KCa), thereby facilitating hyperpolarization, decreased phosphoinositide (PI) hydrolysis, increased Na+/Ca2+ exchange, increased Na+,Ca2+-ATPase activity, and decreased myosin light chain kinase (MLCK) activity. β2 Receptors may also couple to KCa via Gs. PDE, cyclic nucleotide phosphodiesterase.
d. If the asthma attacks occur more frequently, what changes in therapy might be appropriate for this patient?
Short-acting inhaled β2 agonists, such as albuterol, should be used “as required” by symptoms and not on a regular basis in the treatment of mild asthma; increased use indicates the need for more effective anti-inflammatory therapy, that is, corticosteroids. Inhaled corticosteroids (ICSs) are recommended as first-line therapy for all patients with persistent asthma. They should be started in any patient who needs to use a β2 agonist inhaler for symptom control more than twice weekly. They are effective in mild, moderate, and severe asthma and in children as well as adults. ICS may be used in children in the same way as in adults; at doses of 400 μg/d or less, there is no evidence of significant growth suppression. The dose of ICSs should be the minimal dose that controls asthma; once control is achieved, the dose should be slowly reduced.
A 72-year-old woman has chronic difficulty in breathing. She has smoked a pack of cigarettes every day for the last 55 years and was diagnosed as having COPD 15 years ago.
a. What is COPD and how does it differ from asthma?
Chronic obstructive pulmonary disease (COPD) involves inflammation of the respiratory tract with a pattern that differs from that of asthma. In COPD, there is a predominance of neutrophils, macrophages, and cytotoxic T lymphocytes (Tc1 cells). The inflammation predominantly affects small airways, resulting in progressive small airway narrowing and fibrosis (chronic obstructive bronchiolitis) and destruction of the lung parenchyma with destruction of the alveolar walls (emphysema). These pathological changes result in airway closure on expiration, leading to air trapping and hyperinflation, particularly on exercise (dynamic hyperinflation). This accounts for shortness of breath on exertion and exercise limitation that are characteristic symptoms of COPD.
In contrast to asthma, the airflow obstruction of COPD tends to be progressive. The inflammation in the peripheral lung of COPD patients is mediated by multiple inflammatory mediators and cytokines, although the pattern of mediators differs from that of asthma. In marked contrast to asthma, the inflammation in patients with COPD is largely corticosteroid-resistant, and there are currently no effective anti-inflammatory treatments for this disease. In addition to pulmonary disease, many patients with COPD have systemic manifestations (skeletal muscle wasting, weight loss, depression, osteoporosis, anemia) and comorbid diseases (ischemic heart disease, hypertension, congestive heart failure, diabetes). Whether these are due to spillover of inflammatory mediators from the lung or due to common causal mechanisms (such as smoking) is not yet clear, but it may be important to treat the systemic components in the overall management of COPD.
b. What treatments are available for this patient?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree