Overview
Diseases of the lung can be classified into four general categories: (1) obstructive lung disease; (2) restrictive lung disease; (3) infectious disease; and (4) neoplastic disease (Table 13-1). The key clinical difference between obstructive and restrictive lung disease is the forced expiratory volume at one second (FEV1) and the forced vital capacity (FVC) ratio, which is decreased in obstructive lung disease and normal in restrictive lung disease. In obstructive lung disease, air is trapped within the parenchyma; in restrictive lung disease, airway filling is impaired due to fibrosis of alveolar septae. The four main types of obstructive lung disease are emphysema, asthma, bronchiectasis, and chronic bronchitis. Restrictive lung disease can be divided into acute and chronic forms, and chronic forms can be subdivided by etiology (i.e., work related, drug induced, autoimmune, and idiopathic).
Category | Subcategories or Specific Conditions |
|---|---|
Obstructive lung disease | Emphysema Asthma Chronic bronchitis Bronchiectasis |
Restrictive lung disease | Autoimmune Idiopathic Work related Drug related |
Infectious lung disease | Community-acquired typical pneumonia Community-acquired atypical pneumonia Nosocomial pneumonia Aspiration pneumonia Necrotizing pneumonia Chronic pneumonia Pneumonia in immunocompromised |
Neoplastic lung disease | Non–small cell lung carcinoma Small cell lung carcinoma |
The seven major forms of infectious lung disease (i.e., pneumonia) are (1) community-acquired typical (e.g., bacterial); (2) community-acquired atypical (e.g., viral, others); (3) nosocomial; (4) aspiration; (5) necrotizing pneumonia; (6) chronic pneumonia (e.g., fungal, mycobacterial); and (7) pneumonia in immunocompromised hosts. Neoplastic disease can be divided into small cell lung carcinoma and non–small cell lung carcinoma. The designation of non–small cell carcinoma versus small cell carcinoma is of utmost importance when determining treatment options. Small cell carcinoma is assumed at the time of diagnosis to have already metastasized.
This chapter will discuss acute respiratory failure, atelectasis, obstructive lung disease, restrictive lung disease, causes of chronic restrictive lung disease, diffuse pulmonary hemorrhage, pulmonary hypertension, pulmonary infections, pulmonary neoplasms, miscellaneous pleural conditions (including pleural effusions and mesothelioma), and upper respiratory tract conditions.
Acute Respiratory Failure
Overview: There are two types of acute respiratory failure: hypoxemic acute respiratory failure and hypercapnic acute respiratory failure.
Basic description: Respiratory failure with pO2 of < 60 mm Hg.
Causes: Pulmonary edema, acute respiratory distress syndrome (ARDS), pneumonia.
Atelectasis
Overview: Atelectasis is collapse of the pulmonary parenchyma. Because of atelectasis, airways and alveoli are unable to fill, and blood is shunted from the arteries to the veins without adequate oxygenation. The four common types of atelectasis discussed below are compressive, obstructive, microatelectasis, and contraction atelectasis.
Mechanism: A condition or lesion external to the lungs (i.e., in the pleural cavity) compresses the lung and impairs filling of the alveoli upon respiration.
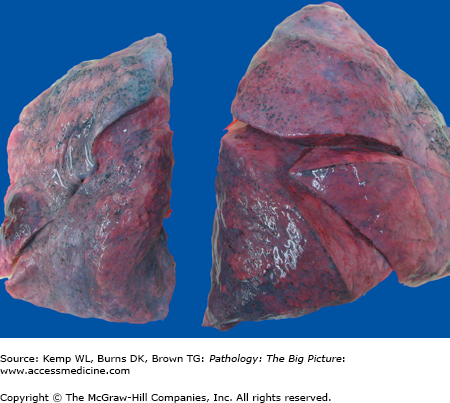
Figure 13-1.
Atelectasis. This photograph shows atelectasis as the result of a left-sided hemothorax due to a gunshot wound. The blood in the left pleural cavity caused compressive atelectasis of the left lung. Note the smaller size of the left lung and its wrinkled pleural surface (due to collapse), compared to the smooth pleural surface of the right lung.
Causes of compressive atelectasis: Blood in the pleural cavity (i.e., hemothorax), air in the pleural cavity (i.e., pneumothorax), and fluid in the pleural cavity (e.g., pulmonary edema).
Mediastinal shift: Away from the source of the atelectasis.
Mechanism: An obstruction in the airway impairs filling of alveoli. All air in the alveoli is eventually resorbed and the alveoli collapse.
Causes of obstructive atelectasis: Aspirated foreign body, tumor, and mucus (e.g., in chronic bronchitis and cystic fibrosis).
Mediastinal shift: Toward the source of the atelectasis.
Obstructive Lung Disease
Overview: Obstructive lung disease is a disease of the lungs that impairs the ability of air to leave the alveoli during expiration, trapping it. It is clinically defined by the decreased FEV1/FVC ratio. The residual volume and functional residual capacity (FRC) are increased, but the total lung capacity may remain normal. The condition eventually leads to hypercapnic respiratory failure, with pCO2 of > 45 mm Hg. The four types of obstructive lung disease discussed below are emphysema, asthma, chronic bronchitis, and bronchiectasis.
Basic description: Disease process that is characterized by the loss of pulmonary parenchyma (i.e., loss of alveolar septae and walls of airways) and dilation of terminal airways.
Types of emphysema
- Centriacinar emphysema, which affects the respiratory bronchioles and involves the upper lobes. Centriacinar emphysema is associated with smoking.
- Panacinar emphysema, which affects the alveoli and alveolar ducts and eventually the respiratory bronchioles and involves the lower lobes. Panacinar emphysema is associated with α1-antitrypsin deficiency.
Mechanism of emphysema: The loss of pulmonary parenchyma causes a loss of elastic recoil. When the patient breathes out, the airways collapse, trapping air because of reduced driving pressure.
Causes of emphysema
- Both centriacinar and panacinar emphysema are caused by an imbalance in protease-antiprotease and oxidant-antioxidant.
- Centriacinar emphysema is caused by cigarette smoking. The nicotine plays several roles.
- Nicotine is a chemoattractant of neutrophils by induction of nuclear factor-κβ and resultant production of tumor necrosis factor (TNF) and interleukin-8 (IL-8). TNF and IL-8 activate neutrophils, which release damaging proteases.
- Nicotine causes inactivation of antiproteases.
- Nicotine causes production of reactive oxygen species, which inactivate proteases and deplete antioxidants.
- Nicotine is a chemoattractant of neutrophils by induction of nuclear factor-κβ and resultant production of tumor necrosis factor (TNF) and interleukin-8 (IL-8). TNF and IL-8 activate neutrophils, which release damaging proteases.
- Panacinar emphysema is caused by a deficiency in α1-antitrypsin. The normal allele encoding α1-antitrypsin is PiMM, but 0.012% of the population has a PiZZ allele, which is associated with a significant decrease in the amount of α1-antitrypsin.
Complications of emphysema
- Pulmonary hypertension as a result of hypoxia-induced vasospasm and loss of vascular surface area (i.e., losing alveolar septae causes loss of alveolar capillaries).
- Cor pulmonale (right-sided heart failure secondary to pulmonary hypertension).
- Mismatched ventilation–perfusion, with shunting of blood to areas of poor ventilation.
Morphology of emphysema: Dilation of airspaces; bullae formation at the pleural surface (Figure 13-2 A–C).
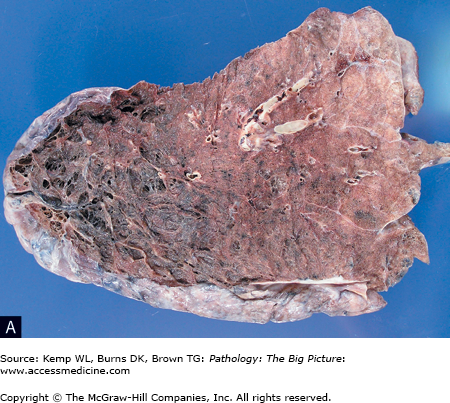
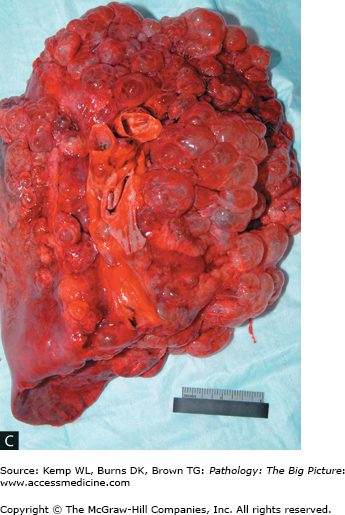
Figure 13-2.
Emphysema. A, The lung is lying on its posterior surface, and the upper lobe is at the left side of the image. Note the loss of parenchyma and greatly increased size of the airspaces (imparting a spiderweb-like appearance). B, The microscopic appearance of emphysema correlates with the gross appearance in A. Once again, note the loss of pulmonary parenchyma and greatly increased size of the airspaces. Hematoxylin and eosin, 40×. C, A lung with marked bullae formation.
Clinical presentation of emphysema
- Signs and symptoms: Dyspnea, hypoxemia, hypercapnia, hyperventilation (patients are referred to as “pink puffers”). Decreased breath sounds and increased expiratory phase on auscultation. Chronic respiratory acidosis with compensatory alkalosis in stable patients. Weight loss (pulmonary cachexia) may be prominent in patients with emphysema, and digital clubbing may be observed.
- Chest radiograph: Flattened diaphragm and expanded hyperlucent lung fields.
- Electrocardiogram: Small amplitude QRS (due to increased airspace) and right axis deviation (usually associated with right ventricular hypertrophy). Tachycardia is common, and multifocal atrial tachycardia (MAT) is classic in patients with COPD.
Basic description: Disease process characterized by episodic reversible bronchoconstriction of hyperreactive airways in response to various exogenous and endogenous stimuli. Asthma is also associated with chronic inflammation.
Types of asthma
- Older classification: Extrinsic and intrinsic.
- Newer preferred classification
- Atopic: A type I hypersensitivity reaction with strong familial tendencies.
- Nonatopic: Asthma associated with viral infection (e.g., rhinovirus, parainfluenza virus) in patients with no family history of allergies and who have normal levels of IgE.
- Drug-induced asthma.
- Occupational asthma.
- Cardiac asthma.
- Atopic: A type I hypersensitivity reaction with strong familial tendencies.
- Alternative classification: Allergic asthma versus nonallergic asthma.
- Allergic asthma
- Epidemiology: Occurs more frequently in children.
- Associated conditions: Patients may have hay fever or eczema.
- Mechanism of allergic asthma: Type I hypersensitivity reaction.
- Causes: Pollens, dust, drugs.
- Epidemiology: Occurs more frequently in children.
- Nonallergic asthma
- Epidemiology: Occurs more frequently in adults.
- Mechanism of nonallergic asthma:Not type I hypersensitivity reaction; IgE levels are normal.
- Causes: Exercise, cold air, drugs, gastroesophageal reflux, viral infections.
- Epidemiology: Occurs more frequently in adults.
Pathogenesis of asthma
- In general, asthma is characterized by hyperreactive airways that constrict in response to stimuli, causing increased airway resistance.
- In atopic and occupational asthma, the disease process is a type I hypersensitivity reaction involving CD4 + TH2 cells, which release IL-4 and IL-5. IL-4 and IL-5 stimulate eosinophils and production of IgE.
- In nonatopic and drug-induced asthma, the mechanism is less well understood, but it is not IgE mediated.
Important point: There are two stages of asthma, early and late.
- Early stage of asthma: Due to the release of mediators from cells, which cause or promote bronchoconstriction (e.g., leukotrienes C4, D4, and E4; histamine, prostaglandin D2 (PGD2). Another mediator released is mast cell tryptase, which inactivates vasoactive intestinal peptide (VIP), a bronchodilator, causing edema and increased vascular permeability.
- Late stage of asthma: The late stage of asthma is due to release of enzymes by eosinophils and neutrophils. The arrival of eosinophils and neutrophils is induced by chemotactic factors released during the early stage of asthma. Neutrophils release proteases, and eosinophils release major basic protein, which are directly toxic to epithelial cells. The late phase is responsible for the morphologic changes that occur in asthma.
Morphology of asthma (Figure 13-3 A–E)
- Gross: Hyperinflated lungs; mucous plugging of airways.
- Microscopic: Hypertrophy of smooth muscle, increased collagen under basement membrane, hyperplasia of mucous glands, and eosinophilic infiltrate; Charcot–Leyden crystals (composed of major basic protein); and Curschmann spirals (i.e., sloughed epithelial cells in mucous cast in the shape of airways).
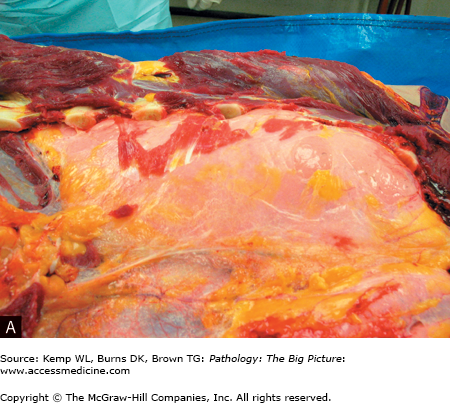
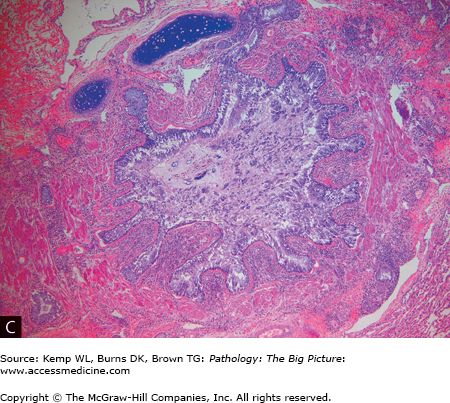
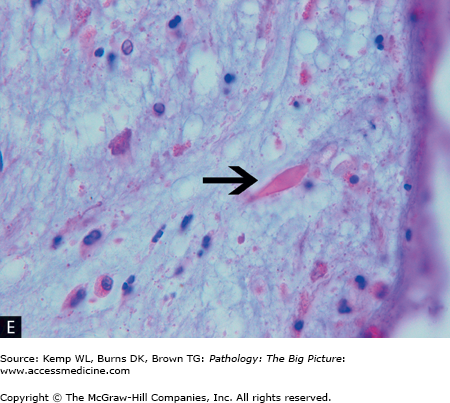
Figure 13-3.
Asthma. A, A patient who died as a result of status asthmaticus. Patients with status asthmaticus can breathe in, but not out. The lungs become overinflated and press against the surrounding chest wall. Note the indentations in the lung produced by its expansion against the ribs. The lung is pink; most lungs, at autopsy, are red and congested from lividity. In this case, however, the pressure on the vasculature produced by the overdistended airspaces prevented blood from settling in the lungs. B, Mucous plugging of the airways (arrowheads), another characteristic gross feature of status asthmaticus. C, Low-power histologic changes associated with asthma, mucous plug of the airway, prominent basement membrane, and smooth muscle hypertrophy. The smooth muscle hypertrophy is producing a vaguely polyp-like architecture to the airway lining, with projections into the lumen. D, The characteristic eosinophilic infiltrate associated with some forms of asthma. E, A Charcot-Leyden crystal (arrow), formed by major basic protein. Hematoxylin and eosin, C, 40×; D, 200×; E, 1000×.
Clinical presentation of asthma
- Symptoms: Classic triad is persistent wheezing, chronic episodic dyspnea, and chronic nonproductive cough. Symptoms may be worse, or only present at night, due to the physiologic drop in cortisol secretion. Night-time cough, which may be the only symptom, is a classic symptom of asthma. Dark rings under the eyes (“allergic shiners”) and a dark transverse crease on the nose (“allergic salute”) are often seen, especially in children. Status asthmaticus is a prolonged asthmatic attack, which can be fatal.
- Laboratory studies: Low peak expiratory flow (PEF). FEV1/FVC is often decreased as in other obstructive lung diseases, and residual volume is increased. Carbon dioxide is usually low in an acute asthma exacerbation secondary to hyperventilation, and a rising carbon dioxide concentration in this setting often precedes respiratory failure. Eosinophilia may be present.
Basic description: Productive cough for at least 3 months in 2 consecutive years.
Pathogenesis: Related to cigarette smoking. Toxins in smoke irritate the airway, resulting in increased production of mucus, which, in turn, stimulates hyperplasia of mucous-secreting glands.
Types of chronic bronchitis: Simple, obstructive, and asthmatic.
Complications of chronic bronchitis
- Obstruction of the airway by mucus, leading to bronchiectasis or atelectasis.
- Pulmonary hypertension.
Morphology of chronic bronchitis
- Gross: Mucous plugging.
- Microscopic: Submucosal gland hypertrophy producing increased Reid index. The Reid index is the thickness of mucous glands in relation to thickness of the wall; in chronic bronchitis, it is > 0.40.
Clinical presentation of chronic bronchitis (see basic description of chronic bronchitis)
- Signs and symptoms: Chronic productive cough; hypercapnia (patients are referred to as “blue bloaters”).
- Important point: Can have asthmatic component (“asthmatic bronchitis”).
Basic description: Abnormal, permanent dilation of airways.
Pathogenesis: Requires two components, infection and obstruction, each one of which can occur first and start the disease process. The infection results in destruction of the smooth muscle and elastic fibers in the wall of the airway.
Causes of bronchiectasis: Allergic bronchopulmonary aspergillosis, cystic fibrosis, and Kartagener syndrome (see related condition below); necrotizing pulmonary infections leading to obstruction (e.g., Staphylococcus, Klebsiella); and other sources of obstruction including tumors, foreign bodies, and mucus in the airways (e.g., from asthma, chronic bronchitis, cystic fibrosis).
Complications of bronchiectasis
- Hemoptysis, with potentially life-threatening hemorrhage.
- Rarely, pulmonary hypertension, abscess formation, and amyloidosis.
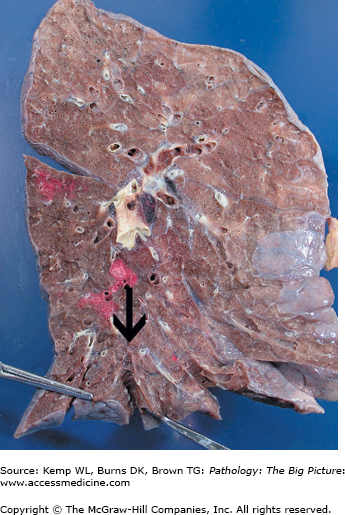
Morphology of bronchiectasis
- Gross: Dilation of airways, usually involving lower lobes, right side more often than left, with airways almost extending to the pleural surface (Figure 13-4).
- Microscopic: Appearance depends upon stage, inflammatory infiltrate, and tissue destruction.
Clinical presentation of bronchiectasis
- Symptoms: Dyspnea, chronic cough (dry, or with large amounts of purulent sputum production). Hemoptysis is common.
- Signs: Clubbing of the fingers (i.e., pulmonary osteoarthropathy), hypoxemia, and hypercapnia.
- Chest radiograph: Parallel lines in peripheral lung fields, which represent nontapering thickened bronchial walls.
Related condition: Primary ciliary dyskinesia
- Genetic abnormality: Hereditary condition associated with short dynein arms.
- Subset of primary ciliary dyskinesia is Kartagener syndrome, which includes bronchiectasis, sinusitis, situs inversus, and sterility.
Chronic Obstructive Pulmonary Disease
Overview: Chronic bronchitis is a clinical diagnosis, and emphysema is an anatomic diagnosis. Patients with symptoms of obstructive lung disease (except asthma and bronchiectasis) are often assigned the clinical diagnosis of chronic obstructive pulmonary disease (COPD). The cause of death in patients with COPD is respiratory acidosis, cor pulmonale, or potentially a pneumothorax.
- Symptoms: Earliest is chronic productive cough, followed by dyspnea on exertion.
- Signs: Increased anteroposterior chest diameter (i.e., barrel chest) due to chronic lung overinflation. Patients use accessory muscles to breath. Patients are often dependent on supplemental oxygen, and pulmonary function tests are consistent with a diagnosis of obstructive lung disease with decreased FEV1/FVC ratio.
Restrictive Lung Disease
Overview: There are two categories of restrictive lung disease, extrapulmonary and intrapulmonary. Extrapulmonary sources include obesity and kyphoscoliosis, and cause a restrictive lung disease by externally impairing filling of the lung. There are two subcategories of intrapulmonary restrictive lung disease, acute and chronic. Acute restrictive lung disease is primarily confined to the diagnosis of acute respiratory distress syndrome (ARDS). Chronic restrictive lung disease is a broad group, which includes many distinct entities. Chronic restrictive lung disease will be discussed following acute restrictive lung disease.
Basic description: Disease developing over a short time period (minutes to days), usually secondary to a major systemic insult (e.g., sepsis, shock), which causes an acute restrictive lung disease, hypoxemic respiratory failure (pO2 is < 60 mm Hg), and diffuse pulmonary infiltrates, and is not attributable to left-sided heart failure. The clinical term for acute restrictive lung disease is acute respiratory distress syndrome (ARDS), and the pathologic term is diffuse alveolar damage.
Pathogenesis of diffuse alveolar damage: Damage to the epithelium or endothelium causes the alveolar septae to become leaky (i.e., increased vascular permeability and loss of diffusion capacity), allowing protein to enter the alveoli. The epithelial cells undergo necrosis and slough into the alveoli. There are three stages of diffuse alveolar damage: exudative, proliferative, and fibrosis.
Stages of diffuse alveolar damage (in order of appearance)
- Exudative stage: The protein and necrotic cells layer out on the alveolar septae, forming hyaline membranes.
- Proliferative stage: Occurs in response to the damage. Type II pneumocytes undergo hyperplasia.
- Fibrosis.
Causes of diffuse alveolar damage
- Four main causes: Severe pulmonary infection, aspiration, sepsis, and severe trauma with shock.
- Other causes: Acute pancreatitis, cardiopulmonary bypass, fat emboli, viral infection (e.g., Hantavirus, severe acute respiratory syndrome [SARS]).
- Acute interstitial pneumonitis (see idiopathic pulmonary fibrosis below) is diffuse alveolar damage of undetermined etiology.
Complications of diffuse alveolar damage: High mortality rate. With survival, patients may develop fibrosis, causing development of a chronic restrictive lung disease, which can lead to pulmonary hypertension.
Morphology of diffuse alveolar damage
- Gross: Firm lungs.
- Microscopic: Hyaline membranes in the exudative stage (Figure 13-5); type II pneumocyte hyperplasia in the proliferative stage; and fibrosis.
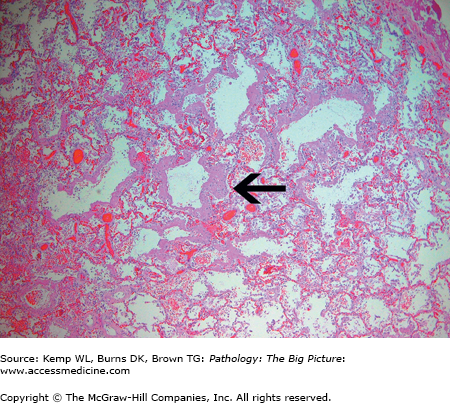
Figure 13-5.
Diffuse alveolar damage. Diffuse alveolar damage (the histologic correlate of the clinical condition, acute respiratory distress syndrome) is characterized by the formation of hyaline membranes (arrow) on the alveolar septae. These hyaline membranes impair oxygen exchanged between the alveoli and alveolar capillaries, producing an acute restrictive lung disease. Hematoxylin and eosin, 40×.
Clinical presentation of diffuse alveolar damage