Gleason grades/score
Usual scenario: primary + secondary patterns
Number of positive cores
Tumor quantitation/extent (percent involvement and/or linear extent in mm)
Treatment-related changes
Table 4.2
Reporting recommendations for prostate cancer variants
Variant | Gleason grade/pattern |
|---|---|
Atrophic | 3 |
Pseudohyperplastic | 3 |
Foamy | 3 or 4 (depending on architecture) |
Vacuoles | 3, 4 or 5 (extract vacuoles/grade architecture) |
Mucinous (colloid) | Either: 4 (based on extracellular mucin alone) or: 3 or 4 (extract mucin/grade architecture) |
Ductal | 4a |
Sarcomatoid | 5 (glandular component graded separately) |
Signet ring cell | 5 |
Small cell/neuroendocrine | Not graded |
Squamous | Not graded |
Basaloid | Not graded |
Table 4.3
Reporting recommendations for special Gleason grading scenarios on needle biopsy specimens
Scenario | Recommendation |
|---|---|
Only one grade present (e.g., GG 3) | Double that grade (assign GS 3 + 3 = 6) |
Abundant high-grade cancer (e.g., GG 4) with < 5 % lower-grade cancer | Ignore the lower-grade cancer (assign GS 4 + 4 = 8) |
Smaller focus with mostly GG 4 and few glands of GG 3 | Since GG 3 occupies > 5 %, include lower-grade cancer (assign GS 4 + 3 = 7) |
Abundant GG 3 with any extent of GG 4 | Include the higher grade (assign GS 3 + 4 = 7) |
Three grades (e.g., GG 3, 4 and 5) present | Classify as high grade (assign most common + highest grade) |
NB: multiple cores showing different grades—cores submitted separately and/or with designated location | Assign separate GS to each core |
NB: multiple cores showing different grades—all cores submitted in one container or cores are fragmented | Assign overall GS for the specimen |
Specimen Submission, Gross Description, and Site Designation
Concurrent with the rise of PSA screening and increasingly sensitive imaging techniques, the average number of prostate NB cores has risen from 2 to 6 to 12 over the past 20 years [6]. As such, the primary purpose of NB has shifted from targeting specific areas of concern on DRE to the systematic mapping of the gland for cancer involvement and quantification [6]. In practice, this information is routinely used to determine (a) whether any form of therapy or follow-up is indicated, (b) the type of therapeutic options offered to the patient, including active surveillance, (c) the extent of resection (i.e., nerve-sparing or not) for surgical patients, and (d) the nature and dosing of radiation therapy .
The import of these results conveys clinical significance to the submission, handling, and description of NB cores. Whether needle cores are submitted in two containers (right and left sides) or in separate containers with specific site designations (i.e., right lateral apex, right lateral mid, right lateral base, etc.) is nonuniform among urologists/institutions. However, the importance of knowing the specific location of the biopsy, and therefore the location of detected cancer, is well recognized, as it allows for detailed correlation with clinical and imaging studies and effective treatment planning [6, 7]. On a practical level, processing and pathologic assessment of NB is greatly facilitated if biopsies are separated. Less material is lost when cutting single biopsies, reading biopsies one by one is easier and facilitates identification of minimal foci of cancer [8]. Therefore, when cores are submitted separately or assigned a clear site designation by container, the pathology report should reflect this labeling [9].
Gleason Grading: Background and Historical Context
The modern system for grading PCa emerged from work in the 1960s by Dr. Donald F. Gleason, based on a specimen cohort from the Veterans Administration Cooperative Research Group [10]. Nearly 50 years later, Gleason grading remains novel in that it is based only on the tumor’s architectural pattern (Gleason patterns 1–5) with the sum of the two most common patterns—Gleason score (GS)—conveying the most clinical meaning (see Chap. 2). While additional morphologic descriptors were added to patterns 3, 4, and 5 in subsequent publications, these observations emanate from the era before PSA screening, when most patients presented with palpable and/or advanced disease and prostatic tissue was typically obtained from transurethral resection [9].
Introduction of PSA screening, coupled with the advent of thin NB techniques and expanded sampling has necessitated the diagnosis and grading of PCa on smaller and better characterized samples. Concurrently, a rising case volume and the importance assigned to Gleason grading in modern predictive models [2–4] have led to increased experience in the application of the Gleason system and a gradual evolution in practice.
In 2005, the International Society of Urologic Pathology (ISUP) convened a conference on Gleason grading to address emerging issues based on existing data as well as the personal/institutional experience of a large international group of urologic pathologists. The resulting manuscript serves as a provisional diagnostic guide to modern Gleason grading [11]. Importantly, the modifications to Gleason grading codified in the 2005 ISUP paper represent collective changes introduced over the course of the 1990s and early 2000s based on much-expanded experience with assessment of prostatic NB and modern radical prostatectomy specimens (see Chap. 2) [9].
Needle Biopsy Gleason Grading: Usual Scenarios
Nearly all prostatic carcinomas seen in NB specimens are of the usual (acinar, conventional) type, to which the Gleason system may be applied. Gleason patterns 1–2 (scores 2–4), which require nodular circumscription as a diagnostic criterion, are not easily evaluable in the limited tissue of NB. Due to poor correlation with prostatectomy grade and reproducibility among experts, GS 2–4 should not be diagnosed in these specimens [11]. Conversely, Gleason pattern 5, including single cells, sheet of cells, and comedocarcinoma, is essentially unchanged from its original descriptions [10]. The 2005 ISUP recommendations convey a significant contraction of Gleason pattern 3 and consequent expansion of Gleason pattern 4, with Gleason pattern 3 typically the lowest assigned grade. The most profound impact of these changes has been on grading of prostatic NB, with GS 7 now being the most commonly assigned score [12].
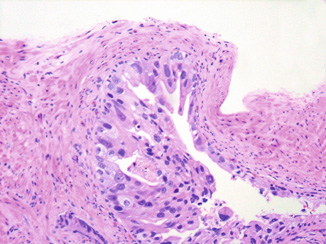
In modern terms, discrete and well-formed, infiltrative glands—even when small—have been retained within Gleason pattern 3. In contrast, practice patterns have evolved with regard to grading of cribriform glands as well as ill-defined glands with poorly formed lumina, originally considered within Gleason pattern 3 [11]. While a percentage of small- to medium-sized cribriform lesions label with basal cell markers and are better recognized today as cribriform high-grade prostatic intraepithelial neoplasia (HGPIN), the remainder of cribriform glands regardless of size, are nearly always diagnosed as pattern 4 [13]. A related feature is glomerulations or glomeruloid structures, characterized by dilated glands with an intraluminal cribriform proliferation attached to the periphery by a “stalk.” This morphology was not accounted for in the original Gleason system and the 2005 ISUP recommendations did not reach consensus on this histology. Nonetheless, a recent report showed that in 45 biopsies with glomerulations, > 80 % showed an association with Gleason pattern 4 cancers in the same biopsy core [14]. The significant morphologic overlap with and occasionally observed transitions to cribriform Gleason pattern 4 carcinoma also favor classifying glomerulations as pattern 4 (Fig. 4.1).
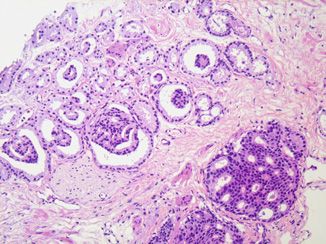

Fig. 4.1
Glomerulations demonstrating significant morphologic overlap with and transition to cribriform Gleason pattern 4 carcinoma
The 2005 ISUP conference highlighted the controversy surrounding classification of “ill-defined glands with poorly formed glandular lumina” (Fig. 4.2). While there is some consensus that such foci should be graded as pattern 4, this morphology represents a significant challenge for the Gleason system, with few instructive images in the existing literature. The ISUP panel cautioned that a “cluster of ill-defined glands in which a tangential section of pattern 3 glands cannot account for the histology ” would be diagnosable as Gleason pattern 4 [11], a determination that necessitates evaluation of multiple levels and sections of such glands.
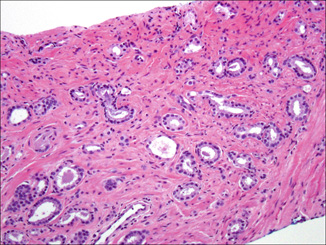

Fig. 4.2
Gleason score 3 + 4 = 7 carcinoma: note multiple poorly formed glands with ill-defined lumina and/or incomplete nuclear complement
Needle Biopsy Gleason Grading: Special Scenarios
Although Gleason grading is and always has been fundamentally based upon a sum of the first and second most common patterns, uropathologists have evolved reporting strategies for some specific scenarios in which (a) morphologic patterns are not well addressed within the original Gleason system, (b) the classic grading might not be clinically precise, and (c) the patient has received prior therapy. While some of these recommendations are consonant with the original Gleason system, the method of applying these rules has been clarified over time. Tables 4.2 and 4.3 summarize these recommendations.
Prostate Cancer Variants
The Gleason grading of a number of variants has been modified from the original system, as reflected in Table 4.2. While the unique clinical and histologic features of PCa variants are dealt with elsewhere in this text, a number of morphologies remain controversial with regard to Gleason grading [15]. Two examples are (a) the group of mucin-related tumors, including carcinomas associated with extravasated mucin (either focal or abundant) and/or mucinous fibroplasia and (b) prostatic ductal adenocarcinoma—especially those cases exhibiting “HGPIN-like” features.
In the NB context, it is difficult to evaluate true mucinous (colloid) carcinoma which requires the presence of > 25 % mucin pools for its diagnosis. However, carcinomas with mucinous features may be diagnosed, typically comprised of irregular cribriform glands in a mucinous background. Such cases may also show individual glands in the same background or simulated “gland within gland” patterns representing single distorted acini and caused by encroachment of acellular mucin in and adjacent to neoplastic glands (Fig. 4.3). A similar finding is carcinoma with mucinous fibroplasia (collagenous micronodules), indicating the delicate ingrowth of fibrous tissue in and among glands, which may result in “fused-” or “cribriform-”appearing glands.
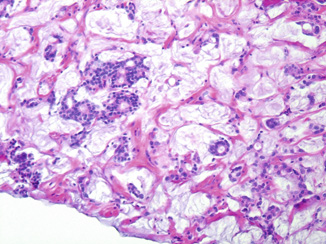

Fig. 4.3
Carcinoma with mucinous features: note that although some truly fused glands (pattern 4) are present, much of the cancer consists of discrete glands (pattern 3) with varying degrees of distortion by extravasated mucin
While mucinous (colloid) carcinoma with cribriform glands is routinely graded as GS 4 + 4 = 8 in radical prostatectomy specimens , there is no consensus regarding cases with discrete glands in a background of extravasated mucin or mucinous fibroplasia. At the 2005 ISUP conference, some suggested that the mucin or mucinous fibroplasia be extracted and the underlying architecture graded [11]. As such, a percentage of these cases would be assigned Gleason pattern 3. Such a designation may be supported by contemporary studies of mucinous carcinoma which have recognized the variability in the epithelial component and reported no death from disease and limited biochemical recurrence without clinical evidence of local or distant recurrence in patients who had mucinous carcinoma treated by RP [16].
The application of the Gleason grading system to prostatic ductal adenocarcinoma has also been controversial, with some initially advocating for not assigning a Gleason grade . The ISUP 2005 recommendations, recognizing that the majority of ductal adenocarcinoma displays complex papillary and/or cribriform morphology, advocated reporting such cases as Gleason pattern 4 with ductal features [11]. More recently, however, a significantly less frequent pattern of ductal adenocarcinoma, characterized by individual glands lined by tall pseudostratified columnar cells has been highlighted among a spectrum of PCa with nuclear stratification in single glands, so-called “prostatic intraepithelial neoplasia (PIN)-like” carcinoma [17]. As these cases may be more frequently associated with usual Gleason pattern 3 PCa and behave in a more indolent fashion than classic ductal adenocarcinoma, some have advocated assigning Gleason grade 3.
Increasing Clinical Precision with Gleason Grading on Needle Biopsy
There are circumstances in which reporting primary + secondary Gleason grade , may be inexact as traditional Gleason grading is unlikely to be representative of cancer in the gland (Table 4.3). In the context of abundant high-grade cancer, for example, lower-grade patterns should not be incorporated in the GS. Hence, a 15 mm core with 13 mm of cancer in which 0.5 mm displays Gleason pattern 3 and the remainder is Gleason pattern 4, should be diagnosed as GS 4 + 4 = 8 [16]. Conversely, any amount of high-grade tumor should be included, as it often reflects more significant high-grade tumor in the gland. As such, a 15 mm core with 13 mm of cancer in which 0.5 mm displays Gleason pattern 4 and the remainder is Gleason pattern 3, should be diagnosed as GS 3 + 4 = 7 [11]. Importantly, to apply the second rule correctly, the possibility of tangential sectioning of Gleason pattern 3 glands masquerading as fused or poorly formed glands must be excluded.
Although Gleason noted the presence of more than two patterns in ~ 50 % of RP specimens, how to address tertiary Gleason patterns in the NB context is controversial, as incorporation of the third most common pattern is by definition contrary to Gleason’s original approach [10]. Nonetheless, in 1.5–4 % of cases, the pathologist encounters a core with three patterns of cancer, most typically, patterns 3, 4, and 5 (e.g., 3 + 4 = 7 or 4 + 3 = 7 with a minor component of 5) [9]. The 2005 ISUP group recommended that such cases be overall classified as high grade (primary grade + highest grade), due to the possibility that the highest grade is a more significant component in the gland and so that the highest grade would be utilized by clinicians when assessing risk using a variety of predictive models, which only allow for two grades. For example, a core with 10 mm of cancer comprised of 65 % pattern 3, 25 % pattern 4, and 10 % pattern 5, would be diagnosed as GS 3 + 5 = 8 [11]. A subsequent NB study has supported this “first + worst” approach, finding earlier time to and percentage of patients with biochemical recurrence in patients with GS 7 with tertiary pattern 5 compared with GS 7 alone [18].
When NB cores are submitted in separate containers and/or have a clearly designated location, the pathologist should assign a separate GS to each sampled core, rather than an overall or averaged score for the entire biopsy session [11, 19]. Such practice avoids weakening the predictive power of the highest GS (e.g., one core showing 4 + 4 = 8 and multiple cores showing 3 + 3 = 6; overall GS would assign 3 + 4 = 7) and is buoyed by studies demonstrating that the highest GS in a specimen correlates with grade and stage at RP [20]. There is no uniform manner of grading cores of differing GS when multiple cores are submitted in one container without site designations. These settings are problematic as the relationship of each core/fragment to another is unclear and the potential for over-grading is increased. So as not to impose a seemingly precise assessment in an inherently imprecise scenario, logic dictates that the pathologist would assign an overall GS in these cases.
Grading Irradiated Cancer
Radiation therapy (external beam and/or brachytherapy [“seeds”]) is commonly used to treat clinically localized or locally advanced PCa . In the setting of a rising PSA postradiation therapy, a biopsy is performed to distinguish local recurrence from metastatic disease and for histological confirmation if salvage RP is to be attempted. Occasionally, the pathologist is not informed of a radiation therapy history and it is therefore essential to recognize the changes in benign and malignant glands that occur with this intervention, which have been well-described elsewhere [21].
While in the past, benign tissue with marked therapeutic effect may have been diagnosed as atypical, increased recognition that these changes are therapy-related has aided their correct identification. Cancerous foci exhibiting profound treatment effect secondary to radiation typically display infiltrative poorly formed glands or single cells with moderate-to-abundant vacuolated clear cytoplasm and prominent nucleoli [21] (Fig. 4.4). When only irradiated cancer is seen, the case may be signed out as “adenocarcinoma with profound treatment effect” and not graded. When usual-type PCa is solely present post-therapy, such that the observed cancer is indistinguishable from that of a patient who had not received radiation, the cancer is graded . In cases in which both gradable cancer and cancer with treatment effect are seen, a reasonable approach is to assign a GS and add a note stating that “the assigned Gleason score reflects the gradable portion of the carcinoma (%); the remaining cancer shows profound treatment effect” [9].
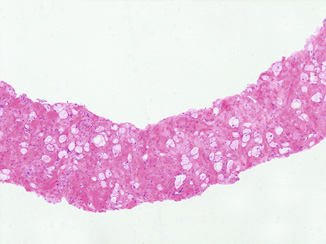

Fig. 4.4
Adenocarcinoma with profound radiation treatment effect: note poorly formed glands and single cells with vacuolated clear cytoplasm
Determining whether “gradable” cancer is present is crucial for clinical management as studies of postradiation NB with 10 years follow-up indicate that the biochemical recurrence-free and distant failure rates for patients having only cancer with profound treatment effect are similar to those with benign NB as opposed to patients with gradable cancer [22]. In other words, the presence or absence of gradable cancer in a postradiation therapy NB is a major indicator of clinical outcome .
Needle Biopsy Gleason Grade as a Measure of Risk
Accumulated evidence from over 40 years of application has shown the biopsy GS to be the most significant predictor of pathologic outcomes at RP, as well as one of the key predictors of clinical outcomes post-RP and radiation therapy [2−5, 23, 24]. GS on NB may also be used to determine therapeutic choices, the extent of neurovascular bundle resection or performance of a pelvic lymph node (LN) dissection . Consequent with the evolution described above, the value of grouping GS (i.e., GS £ 6, 7, 8–10), such that each group behaved worse than the group below it, was recognized. Further substratification of GS 7 based on primary grade (i.e., GS 3 + 4 = 7 vs. 4 + 3 = 7) has also been shown to influence pathologic and clinical outcomes [25] and is routinely reported. More controversy exists as to whether GS 8–10 should be considered a homogenous group, as one study suggests that PCa with NB GS 9–10 are associated with a much worse prognosis than GS 8 [26]. Since GS is incorporated into every predictive tool [2–4] that has been designed for PCa, the accurate and reproducible application of this system has clinical meaning. Major educational efforts in the past two decades have resulted in significantly better correlation for GS on biopsy between community and academic pathologists [23].
Since the 2005 ISUP conference, few formal studies have evaluated its impact [12, 27] and these have been small cohorts with limited follow-up. As many of the “changes” represent modifications by groups of pathologists over time, such an exercise may not be fruitful, as using 2005 as a dividing line between an “old” and “new” system may be biased and inaccurate [9]. These studies have generally documented a higher percentage of NB specimens with GS 7 in post-2005 cohorts, as well as somewhat improved biopsy–prostatectomy GS correlation and prediction of biochemical-free progression after RP.
Extent of Tumor Involvement
For core biopsy specimens, the absolute number of cores examined and involved is routinely reported. In cases with one core submitted per container, this assessment is simple. In the event of multiple cores per container, the degree to which tissue fragmentation has taken place will impact this determination.
Once a diagnosis of cancer has been rendered for a given NB core, there are multiple measures of tumor quantification which have been reported to correlate with pathologic grade and stage as well as to predict biochemical recurrence [28, 29]. However, many of these evaluations are tedious, not routinely used in contemporary practice and may add little to the predictive accuracy of more simple measurements. On the other hand, there is overwhelming consensus that in addition to the number of cores involved, some quantitation of tumor extent on a per core basis should occur, whether by visual estimation of linear extent in millimeters, percentage of core involvement, or both. When multiple cores are submitted in the same cassette, there is a higher likelihood of fragmentation [8] and it may be most prudent to report the percentage of the overall fragmented specimen involved by cancer in these cases.
Within a given NB core, foci of cancer may be present continuously or discontinuously along the length of the specimen. In the former case, length in millimeters and/or percentage of core involvement is readily assessed. When multiple foci of carcinoma are separated by intervening benign prostatic glands and stroma some pathologists will “collapse” the tumor by disregarding the intervening tissue [28] while others will measure the farthest distance between the outer-most foci and report the entire length or percentage as if it was one unbroken focus (e.g., three small foci of carcinoma discontinuously involving 80 % of the core) [29]. This may result in vastly differing tumor quantitation, which may impact nomogram predictions or eligibility for active surveillance. Two contemporary studies of this specific issue convey different findings. The first study showed that in cores with discontinuous foci of cancer, stratifying the cancer lengths by various cutoffs of intervening stroma, below which discontinuous foci would be measured as one focus, yielded equal prognostic significance [28]. In contrast, another report has suggested that for cancer-bearing cores in which the NB GS is reflective of the entire tumor in the RP specimen, quantitating discontinuous foci as one unbroken focus correlates better with pathologic outcomes [29]. Given the limited evidence, it is not possible to draw a definitive conclusion at this time.
Perineural Invasion
Perineural invasion—defined as cancer tracking along or circumferentially around a nerve—is a relatively ubiquitous finding in RP specimens. In NB, an incidence between 11 and 38 % has been reported [30]. There appears to be functional bidirectional communication between nerves and prostatic carcinoma cells accounting, at least in part, for perineural growth which is a major route of extraprostatic extension. Significant controversy exists as to whether this finding on NB predicts extraprostatic spread at RP and/or biochemical recurrence post-therapy (surgical or radiation). Reviews by Bismar et al. and Harnden et al. reveal that while most studies find perineural invasion to be predictive of extraprostatic extension in univariate analysis, its importance is not retained once PSA, clinical stage, and biopsy GS (common preoperative parameters) are considered in multivariate analysis [30, 31]. Similarly, there are conflicting data as to whether perineural invasion predicts for recurrence after surgery or radiation therapy. Importantly, studies which analyzed perineural invasion in specific patient groups stratified by PSA levels, clinical stage, GS, and/or NB tumor extent have found it to be an independent prognostic factor [30].
Urologic surgeons react in different ways to a report of perineural invasion on NB, with some considering this an indication to abandon nerve-sparing surgery. Recent data, however, suggest that bilateral nerve sparing may be performed without compromising oncologic efficacy in the majority of patients [32]. Taking into account the relative ease of identifying perineural invasion and its proposed significance in at least some patient groups, this finding is routinely reported.
High-Risk Lesions and Putative Precursors
Small Foci of Atypical Glands, Suspicious for Carcinoma
“Atypical small acinar proliferation” (ASAP) and “small focus of atypical glands suspicious for carcinoma (ATYP)” are terms that refer to a focus of acini that is suspicious for cancer but lacks sufficient architectural and/or cytological atypia for a definitive diagnosis. If used correctly, these terms reflect the pathologist’s uncertainty as to whether a given glandular focus can be assigned a cancer diagnosis . It is therefore important that ASAP/ATYP not become a “wastebasket” diagnosis, subsuming a large spectrum of lesions. Rather, it should be a diagnosis of last resort, one in which the pathologist, after careful consideration using H&E criteria and ancillary immunohistochemical studies as appropriate, is unable to arrive at a definitive benign or cancer diagnosis [9].
There are many reasons for a finding of ASAP. Some of the more common struggles include: atypical glands that are few in number, foci with procedural-related crush or fragmentation artifact, crowded glands with minimal cytological atypia, glandular foci associated with significant inflammation, and small acinar foci in which outpouching/tangential sectioning of HGPIN cannot be distinguished from limited cancer adjacent to HGPIN [33].
It is important to recognize atypical foci suspicious for cancer in prostatic needle biopsies due to their association with cancer on repeat biopsies [34]. In this sense, ASAP may be seen as a risk factor for the subsequent finding of cancer, with the existing literature reporting an average 40 % risk of cancer following this diagnosis, a rate has been stable for nearly two decades [34]. In some cases, a focus of atypical glands is closely associated with a focus of high-grade PIN, a phenomenon which seems to carry a risk of cancer on repeat biopsy similar to ASAP. It is incumbent upon the pathologist to communicate to his/her colleagues the clinical import of these findings, so that appropriate follow-up, in the form of early repeat NB, may be performed [9].
High-Grade Prostatic Intraepithelial Neoplasia
Although PIN was first described in the 1960s by McNeal, formal characterization did not occur until the late 1980s when it was first termed “intraductal dysplasia” and quickly evolved to “PIN” [35]. Current evidence from a variety of sources has rendered high-grade PIN the only well-established precursor to prostatic adenocarcinoma [33, 34].
Morphologically, PIN describes architecturally benign prostatic glands lined by atypical cells. After initially being divided into three grades, PIN was more concisely classified as either low grade (approximating grade 1) or high grade (approximating grades 2–3), with prominent nucleoli being the primary distinguishing factor [35]. In the past two decades, however, it has become evident that (a) there is low interobserver reproducibility for a diagnosis of low-grade PIN, with even urologic pathologists having difficulty separating this entity from slight variations of normal prostatic glandular architecture, and (b) low-grade PIN does not convey a significantly increased risk of cancer in follow-up biopsy when compared with an initial benign diagnosis [34]. As a result, the diagnosis of low-grade PIN has largely faded from the pathology-reporting spectrum, such that a diagnosis of PIN today refers to high-grade PIN.
Recent reviews reveal a large range of incidence, from 0 to 24.6 % on initial NB, with no apparent relationship between PIN detection and number of cores sampled, year of sampling or academic versus community practice settings [33, 34]. This wide variation may be partially explained by the subjective nature of evaluating “cytologic atypia,” specifically the presence of prominent nucleoli (how prominent? how many?), as well as multiple histological artifacts (thick sections; fixatives that enhance nucleolar detail). The difficulty in defining “atypia” is highlighted in the responses to a survey of urologic pathologists that inquired as to how prominent/how many nucleoli are required for a PIN diagnosis. “Any visible at ×40 magnification,” “any visible at ×20 magnification,” “any visible regardless of magnification,” “in > 10 % of secretory cells at ×40 magnification,” “in > 10 % of secretory cells at ×20 magnification,” and “in > 10 % of secretory cells regardless of magnification” garnered 16, 17, 19, 11, 9, and 13 % of replies, respectively, demonstrating great variability [36] and indicating the need for more specific diagnostic criteria to increase the reproducibility of a PIN diagnosis .
While the incidence of high-grade PIN does not appear to be dependent on the number of cores sampled, with studies in the 6-core and 12-core eras showing similar variability in PIN detection [34], a significantly decreased incidence of cancer detection following a high-grade PIN diagnosis has been observed [34, 37]. Although the literature reveals a large range of cancer incidence post-high-grade PIN diagnosis (from 2.3 to 100 %), a more careful look reveals an incidence of ~ 50 % in 1990s studies which has dropped to ~ 20 % post-2000. This change approximates the shift toward more extended NB schema, which is now routine practice. Furthermore, recent studies which examine the risk of cancer on re-biopsy following a diagnosis of high-grade PIN compared with that following a benign diagnosis have shown no statistically significant differences [37]. This has led some to propose that early repeat NB is not required for men within 1 year of a PIN diagnosis, especially if there is only one core with high-grade PIN. When the initial biopsy has multifocal (> 1 core with PIN), the risk of cancer on immediate repeat biopsy is about 40 % and justifies repeat NB within the first year [38]. However, the long-term risk of cancer with unifocal HGPIN on initial biopsy remains unknown.
Intraductal Carcinoma
Intraductal carcinoma of the prostate is characterized by a malignant proliferation of epithelial cells conforming to the contours of often-expanded native ducts and/or acini displaying basal cells. Early descriptions from RP specimens drew attention to the fact that in contradistinction to high-grade PIN, intraductal carcinoma is rare in areas away from carcinoma [39]. This dichotomy is also reflected in needle biopsies, where it is rarely seen in the absence of invasive cancer. Further studies revealed associations with high GS and tumor volume, as well as increased rates of extraprostatic extension, seminal vesicle invasion, and recurrence after RP [40, 41]. Based on this evidence, most have argued that intraductal carcinoma is part of the evolution of PCa (a late event) or alternatively an aggressive precursor (which may or may not arise from PIN). Recent follow-up series of NB containing exclusively intraductal carcinoma have shown that the overwhelming number has invasive cancer with GS 7 and pT3 disease at subsequent RP [40]. These associations reveal the critical importance of separating high-grade PIN from intraductal carcinoma on NB.
Diagnosing intraductal carcinoma may be difficult, as its description may overlap with that of high-grade PIN in a given case. Additionally, it should be recognized that unlike ductal carcinoma, a morphologic phenotype/variant, intraductal carcinoma is a growth pattern of cancer, the morphology of which can be acinar or ductal. The most commonly agreed upon distinguishing criteria are those of intraductal foci with dense cribriform to solid masses with or without comedonecrosis. Though not always present, marked nuclear atypia, in the form of striking nucleomegaly, hyperchromasia, and/or overt pleomorphism, has been repeatedly associated with intraductal carcinoma (Fig. 4.5). In practice, identifying rounded or circumscribed masses of malignant cells with complex architecture and/or obvious nuclear atypia and a preserved basal cell layer should raise the diagnostic possibility of intraductal carcinoma [9]. Given the well-established correlation with high-grade, high-stage disease at RP, when detected, the presence of intraductal carcinoma should be noted in NB reports.