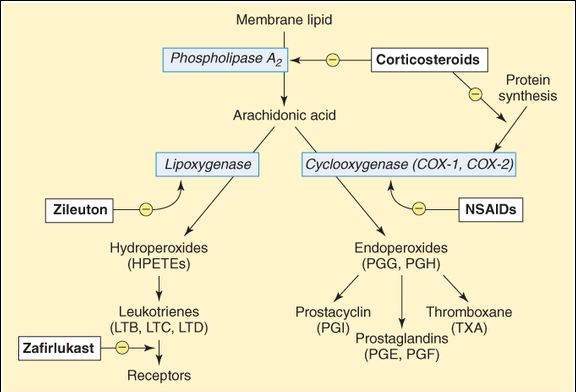
FIGURE 18-1
Synthesis of eicosanoid autacoids. Arachidonic acid is released from membrane lipids by phospholipase A2 and then converted into straight-chain derivatives by lipoxygenase or into cyclized derivatives by cyclooxygenase. Because many of the effects of these products are pathogenic, drugs that inhibit synthesis or prevent the actions of the products are clinically useful.
High-Yield Terms to Learn
Abortifacient A drug used to cause an abortion. Example: prostaglandin F2
Cyclooxygenase Enzyme that converts arachidonic acid to PGG and PGH, the precursors of the prostaglandins Dysmenorrhea Painful uterine cramping caused by prostaglandins released during menstruation Endoperoxide General term for prostaglandin precursors, for example, PGG, PGH Great vessel transposition Congenital anomaly in which the pulmonary artery exits from the left ventricle and the aorta from the right ventricle. Incompatible with life after birth unless a large patent ductus or ventricular septal defect is present Lipoxygenase Enzyme that converts arachidonic acid to leukotriene precursors (HPETEs) NSAID Nonsteroidal anti-inflammatory drug, for example, aspirin, ibuprofen, celecoxib. Inhibitor of cyclooxygenase Oxytocic A substance that causes uterine contraction Patent ductus arteriosus Persistence after birth of the fetal shunt between the pulmonary artery and the aorta Phospholipase A2 Enzyme in the cell membrane that generates arachidonic acid from membrane lipid constituents Slow-reacting substance of anaphylaxis (SRS-A) Material originally identified by bioassay from tissues of animals in anaphylactic shock; now recognized as a mixture of leukotrienes, especially LTC4, and LTD4
Eicosanoid Agonists
Classification
The principal eicosanoid subgroups are the straight-chain leukotrienes and the cyclic molecules, including prostaglandins, prostacyclin, and thromboxane. The leukotrienes retain the straight-chain configuration of arachidonic acid. Prostacyclin, thromboxane, and other members of the prostaglandin group are cyclized derivatives of arachidonic acid. There are several series for most of the principal subgroups, based on different substituents (indicated by letters A, B, etc) and different numbers of double bonds (indicated by a subscript number) in the molecule.
Synthesis
Active eicosanoids are synthesized in response to a wide variety of stimuli (eg, physical injury, immune reactions). These stimuli activate phospholipases in the cell membrane or cytoplasm, and arachidonic acid (a tetraenoic [4 double bonds] fatty acid) is released from membrane phospholipids (Figure 18-1). Arachidonic acid is then metabolized by several mechanisms, 2 of which are most important. First, metabolism to straight-chain products is performed by lipoxygenase, ultimately producing leukotrienes. Alternatively, cyclization by the enzyme cyclooxygenase (COX) may occur, ultimately resulting in the production of prostacyclin, prostaglandins, or thromboxane. COX exists in at least 2 forms. COX-1 is found in many tissues; the prostaglandins produced in these tissues by COX-1 appear to be important for a variety of normal physiologic processes (see later discussion). In contrast, COX-2 is found primarily in inflammatory cells; the products of its actions play a major role in tissue injury (eg, inflammation). In contrast to these inflammatory functions, COX-2 is also responsible for synthesis of prostacyclin in the vascular endothelium and of prostaglandins important in renal function. Thromboxane is preferentially synthesized in platelets, whereas prostacyclin, as noted, is synthesized in the endothelial cells of vessels. Naturally occurring eicosanoids have very short half-lives (seconds to minutes) and are inactive when given orally.
Replacement of tetraenoic fatty acids in the diet with trienoic (3 double bonds) or pentaenoic (5 double bonds) precursors results in the synthesis of much less active prostaglandin and leukotriene products. Thus, dietary therapy with fatty oils from plant or cold-water fish sources can be useful in conditions involving eicosanoids.
Mechanism of Action
Most eicosanoid effects appear to be brought about by activation of cell surface receptors (Table 18-1) that are coupled by the Gs protein to adenylyl cyclase (producing cyclic adenosine monophosphate [cAMP]) or by the Gq protein to the phosphatidylinositol cascade (producing inositol 1,4,5-trisphosphate [IP 3] and diacylglycerol [DAG] second messengers).
TABLE 18-1 Effects of some important eicosanoids.
Effect PGE 2
PGF 2
PGI 2
TXA 2
LTB 4
LTC 4
LTD 4
Major receptors EP1-4
FPA,B
IP TP ,
,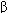
BLT1,2
CysLT2
CysLT1
Coupling protein Gs, Gq
Gq
Gs
Gq
Gq
Gq
Gq, Gi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree