Proliferative Disorders and Carcinoma of the Endometrium
With a marked decrease in the rate of invasive cancer of the uterine cervix, cancer of the endometrium has become the most common cancer of the female genital tract diagnosed in the United States, with the second highest mortality rate after ovary. The death rate from endometrial carcinoma increased substantially between the years 1990
and 2000 (Greenlee et al, 2000). A major increase in the rate of endometrial cancer has also been observed in other countries, such as Japan (Sato et al, 1998) and Canada (Byrne, 1990). Therefore, the primary goal of diagnostic cytology of the endometrium should be the diagnosis of clinically unsuspected endometrial carcinoma of low stage and, hence, amenable to cure. In a study of a large group of asymptomatic women, it has been documented by Koss et al (1981, 1984) that approximately 8 per 1,000 peri- and postmenopausal women harbor such lesions. The study is described in detail further on in this chapter. Prior to this work, primary cytologic diagnosis of occult endometrial carcinoma was rarely reported, particularly when compared with the wealth of material on the uterine cervix. Twenty-two of 102 endometrial cancers, diagnosed in cervicovaginal smears, occurred in asymptomatic women (Koss and Durfee, 1962). In a series of 285 endometrial carcinomas reported by Reagan and Ng (1973), there were only 18 cases with primary diagnosis by cytology. Only a few additional cases may be found in the older case reports, including some illustrated in Papanicolaou’s Atlas (1954). It is quite evident that detection of early endometrial carcinoma has not reached the level of interest equal to detection of mammary or cervical cancer. For whatever reasons, this important disease has been neglected by the society.
and 2000 (Greenlee et al, 2000). A major increase in the rate of endometrial cancer has also been observed in other countries, such as Japan (Sato et al, 1998) and Canada (Byrne, 1990). Therefore, the primary goal of diagnostic cytology of the endometrium should be the diagnosis of clinically unsuspected endometrial carcinoma of low stage and, hence, amenable to cure. In a study of a large group of asymptomatic women, it has been documented by Koss et al (1981, 1984) that approximately 8 per 1,000 peri- and postmenopausal women harbor such lesions. The study is described in detail further on in this chapter. Prior to this work, primary cytologic diagnosis of occult endometrial carcinoma was rarely reported, particularly when compared with the wealth of material on the uterine cervix. Twenty-two of 102 endometrial cancers, diagnosed in cervicovaginal smears, occurred in asymptomatic women (Koss and Durfee, 1962). In a series of 285 endometrial carcinomas reported by Reagan and Ng (1973), there were only 18 cases with primary diagnosis by cytology. Only a few additional cases may be found in the older case reports, including some illustrated in Papanicolaou’s Atlas (1954). It is quite evident that detection of early endometrial carcinoma has not reached the level of interest equal to detection of mammary or cervical cancer. For whatever reasons, this important disease has been neglected by the society.
Endometrial cytology belongs to the most difficult areas of morphology. There are two main reasons for it:
The difficulties with obtaining a representative sample of the endometrium
The difficulties in the interpretation of the cytologic evidence and the recognition of normal and abnormal cells of endometrial origin
This chapter is dedicated to the description of endometrial cytology in health and disease, compared with histologic observations.
CYTOLOGY OF ENDOMETRIUM IN HEALTH AND BENIGN CONDITIONS
Routine Cervicovaginal Samples
The recognition of normal glandular and stromal endometrial cells in routine cervicovaginal samples plays a critical role in the diagnosis of endometrial abnormalities. Therefore, a brief recall of commonly observed cytologic findings is summarized here.
Normal Findings
Childbearing Age
As described and illustrated in Chapter 8, glandular and stromal endometrial cells are normally found in routine cervicovaginal samples during menstrual bleeding and for 2 to 3 days thereafter. As a rule, the finding of endometrial cells, regardless of morphology, after the 12th day of the cycle (considering the first day of bleeding as the first day of the cycle) must be considered abnormal. Depending on the clinical situation (e.g., patient’s age, clinical history, risk factors for endometrial cancer; see discussion below), the patient may be deserving of follow-up or further investigation, although, in most such women, no significant lesions are found and the endometrial cells are most likely a variant of normal shedding.
In endocervical brush specimens, normal endometrial cells, derived from the lower uterine segment (LUS) of the endometrial cavity, may be observed, regardless of day of cycle, and should not be a cause for alarm, although incidental endometrial abnormalities may sometimes be recognized in such samples (see below). De Peralta-Venturino et al (1995) and Heaton et al (1996) stressed that material obtained from LUS may contain large fragments of endometrial glands and stroma that may be mistaken for carcinomas of endometrial or endocervical origin and benign entities, such as endometriosis.
Menopause
In postmenopausal women, the presence of endometrial cells in routine smears must be considered, a priori, abnormal and calls for further investigation of the endometrium.
Benign Conditions and Disorders
Pregnancy
Endometrial cells are practically never seen in normal pregnancy. The decidual cells and particularly the large Arias-Stella cells with dark, polyploid nuclei, either derived from the endometrium or the endocervix, both discussed and illustrated in Chapter 8, may be mistaken for endometrial cancer cells in cervicovaginal material. Pregnancy does not rule out endometrial cancer. On the rarest occasion, we have observed normal pregnancy occurring in women with endometrial carcinoma documented by prior biopsy and confirmed postpartum. A similar case was described by Kowalczyk et al (1999) who also summarized the very scanty literature on this topic. Apparently, normal implantation of the ovum may occur under these circumstances. Also on record are several cases of normal pregnancies occurring in women with documented endometrial hyperplasia (Kurman et al, 1985).
Intrauterine Contraceptive Devices
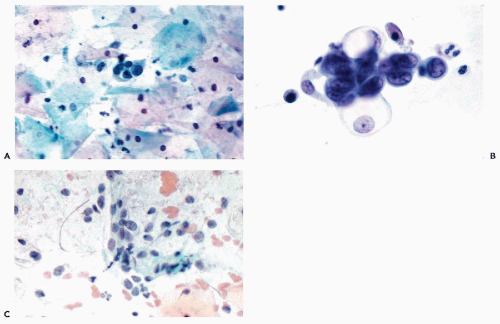
As has been described in Chapters 8 and 10, the wearers of intrauterine contraceptive devices (IUDs) may shed endometrial cells at midcycle. Occasionally, such cells have a vacuolated cytoplasm and poorly preserved nuclei that may appear to be somewhat enlarged and slightly hyperchromatic and that may be mistaken for cells of an adenocarcinoma (Fig. 13-1). Sometimes, the cervicovaginal smears may also contain inflammatory cells and macrophages, creating a cytologic background, not unlike that seen in endometrial carcinoma (see below). The young age of most wearers of IUDs is usually against this latter diagnosis. Another potential source of error is the presence of endocervical “repair” caused by IUD, in which the reactive endocervical cells may be mistaken for abnormal endometrial cells (see Chapter 10; and comments below).
An important histologic finding in wearers of the IUD
is the presence of small, round foci (morulae) of squamous cells in the superficial layers of the endometrium, presumably a form of squamous metaplasia, induced by the mechanical effect of the devices. Lane et al (1974) suggested that this abnormality is transient, although evidence of reversal of this process is poor. These abnormalities are very rarely seen and should not be mistaken for an endometrioid carcinoma with squamous component or an adenoacanthoma (see below).
is the presence of small, round foci (morulae) of squamous cells in the superficial layers of the endometrium, presumably a form of squamous metaplasia, induced by the mechanical effect of the devices. Lane et al (1974) suggested that this abnormality is transient, although evidence of reversal of this process is poor. These abnormalities are very rarely seen and should not be mistaken for an endometrioid carcinoma with squamous component or an adenoacanthoma (see below).
Signet-Ring Cells
Iezzoni and Mills (2001) described 5 symptomatic patients in whom routine endometrial tissue samples contained aggregates of benign signet ring cells with small nuclei. The authors traced these cells to decidualized stromal cells. There is no record of such cells in cytologic samples.
Endometrial Metaplasia
Johnson and Kini (1996) described the presence of atypical endometrial cells in the presence of eosinophilic, papillary, squamous and tubal metaplasia of the endometrium. Five of seven patients were postmenopausal and three had abnormal bleeding. The nature of this observation is questionable and it cannot be excluded that some of the patients had a poorly defined neoplastic process.
Exogenous Hormones
Contraceptive Hormones
Women receiving this medication occasionally bleed or spot and shed endometrium at mid-cycle (breakthrough bleeding) until the dosage is adjusted. Long-term usage of these agents may result in decidua-like changes in endometrial stroma, followed by atrophy; neither of these conditions is known to cause endometrial shedding. Abnormalities of nuclei of endocervical cells may occur in women receiving progesterone-rich contraceptive agents (see Chapter 10). Accurate clinical history is helpful in preventing errors but, in some cases, may require biopsies for clarification.
Steroid Hormones
In patients receiving steroid hormones, particularly estrogens, two important cytologic changes may be observed.
In postmenopausal women, the level of maturation of the squamous cells may increase (see Chapter 9), resulting in a smear pattern that is sometimes seen in endometrial hyperplasia and early endometrial carcinoma (see below).
The patients may shed endometrial cells during medication and, particularly, immediately after withdrawal of estrogens (withdrawal bleeding). In the absence of clinical data in postmenopausal women, the presence of endometrial cells may cause an unnecessary alarm. The potential carcinogenic effects of estrogens and tamoxifen are discussed below, in conjunction with epidemiology of endometrial carcinoma.
For further comments on effects of steroid hormones, see Chapter 9.
Regenerating Endometrium
Following a curettage or other form of trauma to the endometrium, the healing of the endometrial defect leads to an intensive proliferation of the surface epithelium, followed by formation of endometrial glands by invagination of the surface epithelium. In histologic sections, the surface epithelium is composed of large cells of variable sizes with hyperchromatic nuclei, sometimes with large nucleoli, and with numerous mitoses. In endometrial aspiration smears, the large and poorly preserved endometrial glandular cells have a vacuolated cytoplasm, sometimes infiltrated with polymorphonuclear leukocytes and enlarged hyperchromatic nuclei (Fig. 13-2). These cells may be mistaken for cancer cells. In this situation, it is advisable to wait until after a normal menstrual bleeding has taken place (usually about 6 weeks after the procedure) before attempting to judge the status of the endometrium.
Inflammatory Lesions
Purulent endometritis resulting from bacterial infection may follow childbirth or abortion. The cervicovaginal smears may disclose pus and debris. Smears obtained by direct endometrial sampling show acute inflammation and necrosis. Fragments of endometrial glands with degenerated, blown-up cells may be difficult to distinguish from cells of necrotizing endometrial carcinoma. The differential diagnosis may have to rest on clinical history and histologic evidence.
Chronic nonspecific endometritis is an uncommon condition in which there is an infiltration of the endometrium by lymphocytes, plasma cells, and macrophages, sometimes with atrophy of the glands. The condition is virtually never recognized in cytologic samples.
Tuberculosis of the Endometrium
A resurgence of tuberculosis in patients with immune deficiency caused by AIDS has revived interest in this disease in the developed countries. The disease is common in the developing world.
Histology
Advanced tuberculosis of the endometrium may be associated with a marked disruption of the endometrial gland pattern. Atypical glandular proliferation may be very
marked and misleading to the point of suggesting a carcinoma. Only the presence of granulomas identifies the condition. The diagnosis should be confirmed by demonstration of tubercle bacilli. The clinical presentation of endometrial tuberculosis is not helpful because the symptoms, such as metrorrhagia, may suggest cancer clinically.
marked and misleading to the point of suggesting a carcinoma. Only the presence of granulomas identifies the condition. The diagnosis should be confirmed by demonstration of tubercle bacilli. The clinical presentation of endometrial tuberculosis is not helpful because the symptoms, such as metrorrhagia, may suggest cancer clinically.
Cytology
The abnormalities of the endometrial glands are also reflected in cervicovaginal smears. Sheets of large endometrial glandular cells of uneven size and with pronounced nuclear hyperchromasia may suggest endometrial cancer (Fig. 13-3). In such cases, the differential diagnosis between tuberculosis and endometrial carcinoma may prove to be extremely difficult, if not impossible, on cytologic grounds. To our knowledge, neither epithelioid cells nor Langhans’-type giant cells have been so far identified in endometrial material as they have been in cervical smears (see Chap. 10). The presence of multinucleated histiocytes in the cervicovaginal smears is of no diagnostic value in the diagnosis of tuberculosis.
Sarcoidosis
This granulomatous disease of unknown etiology may affect the endometrium (Chalvardijian, 1978; Skehan and McKenna, 1986; Elstein et al, 1994). Noncaseating granulomas, characteristic of this disorder, are observed in histologic material but, so far, have not been observed in cytologic material. For a description of cytologic presentation of pulmonary sarcoidosis, see Chapter 19.
Viral Endometritis
Astin and Askin (1975) and Wenckebach and Curry (1976) described endometritis due to cytomegalovirus. The tissue showed evidence of chronic inflammation and formation of lymphocytic deposits, in addition to large cells containing the characteristic viral inclusions. Wenckebach and Curry confirmed the diagnosis by electron microscopy. Duncan et al (1989) described a case of necrotizing endometritis
associated with herpesvirus infection. Neither of these viral infections of the endometrium have been reported in cytologic writing.
associated with herpesvirus infection. Neither of these viral infections of the endometrium have been reported in cytologic writing.
Other Inflammatory Disorders
A case of malacoplakia was described by Thomas et al (1978). For further comments on histologic and cytologic presentation of malacoplakia, see Chapter 22.
Cytologic Atypias Associated With Endometriosis
Several observers reported that brush samples in cases of endocervical or transformation zone endometriosis may contain abnormal glandular cells that may mimic either an endocervical or an endometrial carcinoma (Hanau et al, 1997; Mulvany and Surtees, 1999; Lundeen et al, 2002).
The abnormalities allegedly caused by endometriosis were illustrated in Figure 11-35C, as examples of atypical glandular cells of unknown significance. In the judgment of this writer, cytologic diagnosis of endometriosis cannot be established. The changes described are most likely brushartifacts with inadequate correlation with histologic findings.
Endometrial Abnormalities Associated With Uterine Leiomyomas
Leiomyomas are by far the most common benign tumors of the uterine corpus. The tumors, composed of bundles of smooth muscle and connective tissue, richly supplied with blood vessels, are often multiples and may reach large sizes. Hemorrhagic necrosis or infarction are known complications of leiomyomas. Many women with benign leiomyomas of the uterus experience episodes of abnormal uterine bleeding. The bleeding is attributed to various causes, such as the inability of the uterus to contract because of interference of leiomyoma with myometrial functions, or to submucosal position of the leiomyoma, causing focal ulceration of the endometrium. Objective evidence for these events is conspicuously absent. However, there is evidence that, at least in some women, the bleeding may be caused by endometrial hyperplasia, which is present in about 50% of women with leiomyomas (Deligdisch and Loewenthal, 1970). Both these disorders (hyperplasia and leiomyomas) may have a common denominator, namely, hormonal imbalance due to preponderance of estrogens. In such cases, the cytologic presentation is similar to other forms of endometrial hyperplasia (see below).
ENDOMETRIAL POLYPS
Benign endometrial polyps may occur in any adult woman but are more common in the fifth decade of life and are a known cause of abnormal uterine bleeding and endometrial shedding. The tumors may originate in any part of the endometrial cavity and may vary in diameter from a few millimeters to several centimeters. The polyps, which may be single or multiple, may be broad-based or pedunculated and sometimes may protrude through the external os of the uterine cervix. Atypia of endometrial glands is common in polyps and may account for abnormalities of endometrial cells in direct endometrial samples (see below). Also, endometrial carcinomas may originate in polyps. The uncommon mesodermal mixed tumors of endometrium may originate in or mimic endometrial polyps (see Chapter 17).
Histology
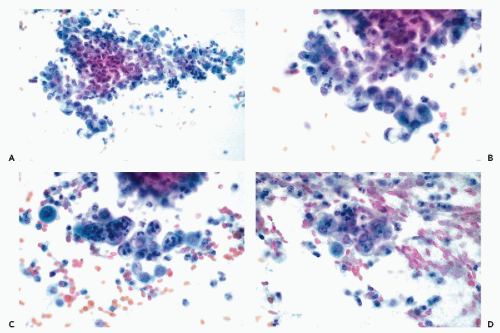
The benign polyps consist of a stroma resembling normal endometrial stroma intermingled with connective tissue that is sometimes hyalinized. The polyps are sometimes richly vascularized, with vessels present near the surface. The epithelial surface lining usually resembles proliferative endometrium but, in polyps originating in the lower uterine segment, it is occasionally composed of columnar cells, resembling normal endocervical lining. Occasionally, the epithelial cells are ciliated. Endometrial glands of variable sizes and shapes are present within the stroma. The epithelial lining of the glands is usually nonsecretory in type and does not participate in the cyclic changes. Atypical endometrial glands, lined by cells with enlarged nuclei and nucleoli mimicking glands observed in atypical hyperplasia, are fairly common in polyps (Fig 13-4D).
Cytology
An accurate cytologic diagnosis of an endometrial polyp is impossible in cervicovaginal samples. Occasionally, clusters or single endometrial cells are noted during the secretory phase of the cycle when endometrial cells should not be present, or in postmenopausal women (Fig. 13-4A-C). In postmenopausal women, the cytologic findings may be mistaken for an endometrial carcinoma. This error is unavoidable. Abnormalities mimicking carcinoma are also observed in direct endometrial samples, as described in detail below.
Large, protruding polyps, pressing on the endocervical epithelium, may elicit a florid squamous metaplasia or “repair” reaction (see Chapter 10). Endometrial carcinomas, originating in polyps, have the same cytologic presentation as primary endometrial cancer (see below).
Atypical polypoid adenomyoma is a rare and presumably benign type of endometrial polyp wherein markedly atypical proliferation of endometrial glands may occur (summary in Young et al, 1986). The possibility that these lesions represent an early stage of a mesodermal mixed tumor cannot be ruled out (see Chapter 17). There is no information on their cytologic presentation.
ENDOMETRIAL ADENOCARCINOMA
As described in the opening paragraphs of this chapter, endometrial carcinoma is, at the time of this writing (2004), the most common form of genital cancer. Partridge et al (1996) observed that the mortality rate from this disease is high and that advancing age, minority status, and low income
had a negative impact on survival. These authors deplored the absence of acceptable early detection systems. Such systems do exist, as narrated below, but their implementation and societal acceptance are thoroughly lagging when compared with carcinoma of the uterine cervix and female breast.
had a negative impact on survival. These authors deplored the absence of acceptable early detection systems. Such systems do exist, as narrated below, but their implementation and societal acceptance are thoroughly lagging when compared with carcinoma of the uterine cervix and female breast.
Some of the reasons for a marked increase in the rate of this disease are discussed here.
Epidemiology
The constant growth and disintegration of the endometrium during the menstrual cycles of the childbearing age constitute a terrain that is not favorable to neoplastic growth and accounts for the rarity of endometrial cancer in women prior to menopause. The absence of cyclic desquamation after the menopause or an arrest of endometrial turnover because of hormonal imbalance are important risk factors in the formation of endometrial carcinomas and their precursor lesions. Examples of naturally occurring conditions leading to hormonal imbalances are the Stein-Leventhal syndrome and similar disorders of ovulation (see Chapter 9) or estrogen-producing ovarian tumors (granulosa cell tumors and thecomas). Endometrial carcinoma has also been observed in the presence of ovarian dysfunction associated with masculinizing features (Koss et al, 1964).
Risk Factors
Exogenous Estrogens
In the late 1960s and in the 1970s, a statistically significant increase in the rate of endometrial carcinoma has been observed in many institutions throughout the United States. Smith et al, Ziel and Finkle simultaneously pointed out in 1975 that widespread administration of conjugated and nonconjugated exogenous estrogens to alleviate menopausal symptoms and prevent osteoporosis was statistically associated with this increase. Mack et al (1976) calculated the risk ratio for endometrial carcinoma in estrogen users when compared with nonusers at 8.0 times, and for conjugated estrogens at 5.6 times; these investigators also demonstrated a dose-related effect on endometrial carcinoma. In a study by a writers group for the PEPI Trial (1996), the administration of unopposed estrogens was shown to cause endometrial hyperplasia and occasional adenocarcinoma. The effect could be prevented by the administration of progesterone. Exogenous estrogens have been shown to be associated with endometrial carcinoma, even in the absence of ovarian function, for example in ovarian agenesis (Gray et al, 1970;
Cutler et al, 1972) or in Sheehan’s syndrome (Reid and Shirley, 1974).
Cutler et al, 1972) or in Sheehan’s syndrome (Reid and Shirley, 1974).
Although the evidence is substantial that estrogens may cause endometrial carcinoma, it has been shown that such lesions observed in estrogen-treated patients are usually fully curable, low-grade and low-stage cancers (Robboy et al, 1982). Horwitz and Feinstein (1978) addressed this issue and reported on the status of peripheral endometrium in a case control study of 233 postmenopausal women, 112 of whom had endometrial carcinoma. Peripheral, simple endometrial hyperplasia was more commonly observed with grade 1 cancer among estrogen users than in cancer of higher grades among nonusers of estrogen. The authors concluded that “it was likely that many otherwise asymptomatic tumors might have remained undetected except for the manifestations of the estrogen-related comorbid condition” (hyperplasia). The observation was repeated by Horwitz et al (1981) who proposed that the effect of estrogens on endometrium is indirect: the drugs cause endometrial hyperplasia and, hence, uterine bleeding that leads to curettages and results in incidental discovery of small foci of early endometrial cancer. In fact, in our own study of occult endometrial carcinomas, estrogen treatment has not been shown to be a risk factor except for women with lower than average weight. It was hypothesized that this observation may perhaps be explained by the inability of this group of women to store the estrogens in their subcutaneous fat, resulting in more direct action on the endometrium (Koss et al, 1984; see below). The use of either estrogen therapy or estrogens combined with progesterone, also increases the risk of breast cancer (Colditz et al, 1995; Schairer et al, 2000) (see Chap. 29).
Tamoxifen
Tamoxifen is a steroid agent best characterized as an estrogen agonist or estrogen-receptor modulator, which blocks estrogen receptors in a variety of tissues and is now extensively used for prevention and treatment of breast cancer (summary in Osborne, 1998). The drug has several side effects affecting the female genital tract and, specifically, the endometrium.
It induces maturation of squamous cells in postmenopausal women with atrophic genital tract (Athanassiadou et al, 1992; Abadi et al, 2000).
It has a stimulatory effect on the endometrium and has been recognized as a cause of abnormal endometrial proliferative processes, including polyps, hyperplasias, and carcinoma (Silva et al, 1994; Assikis and Jordan, 1995; Barakat, 1996; Fisher et al, 1994). The risk appears to be greater for obese women (Bernstein et al, 1999). Sporadic cases of mesodermal mixed tumors were also observed (Bouchardy et al, 2002; Wysowski et al, 2002; Wickerham et al, 2002). Common sense would suggest that the status of the endometrium should be determined in all women prior to tamoxifen therapy.
Measuring the thickness of the endometrium by ultrasound is a favored method of follow-up of patients receiving tamoxifen and other hormones (Achiron et al, 1995; Levine et al, 1995; Hann et al, 1997). It has been suggested that endometrial thickness of 8 mm or more should trigger an endometrial investigation by biopsy or curettage. Langer et al (1997), using the thickness of 5 mm as a trigger for endometrial biopsies in women receiving estrogen replacement therapy, noted that at this level of endometrial thickness, the technique has a very poor positive predictive value but a high negative predictive value for important endometrial disorders.
Cytologic Observations in Tamoxifen Users
The information on the use of cytologic techniques to determine the status of the endometrium in tamoxifen-treated patients is scarce. Yet, anecdotal evidence based on personal observations of a few patients by endometrial sampling has shown that, after a few years of medication, significant nuclear abnormalities may occur in glandular endometrial cells, that differ significantly from patterns of endometrial hyperplasia or carcinoma and most likely represent tamoxifen-induced endometrial atypia (Fig. 13-5). Abadi et al (2000), in a study encompassing a small number of patients treated with tamoxifen, some of whom developed
endometrial carcinoma, noted that the presence of endometrial cells and an increase in macrophages in cervicovaginal smears, correlated in a statistically significant fashion with endometrial cancer.
endometrial carcinoma, noted that the presence of endometrial cells and an increase in macrophages in cervicovaginal smears, correlated in a statistically significant fashion with endometrial cancer.
Other Hormones
Endometrial carcinoma has been observed in approximately 0.05% of women treated with a variety of hormones for carcinoma of the breast (Hoover et al, 1976). Hormonal contraceptive agents usually cause endometrial atrophy. It is not known, at this time, whether these agents may also contribute to the genesis of endometrial cancer, although a few such cases have been recorded (Silverberg and Makowski, 1975).
Radiotherapy
Malignant tumors of the endometrium (carcinomas and occasionally mesodermal mixed tumors) have been observed in patients who received a curative dose of radiation for invasive carcinoma of the uterine cervix (Fehr and Prem, 1974).
Clinical Risk Factors
Carcinoma of the endometrium has been traditionally thought to be associated with diabetes, obesity, hypertension, a past history of abnormal menses, and late menopause (Wynder et al, 1966; Elwood et al, 1977). Our own epidemiologic studies of asymptomatic women with occult carcinoma failed to confirm these observations (Koss et al, 1984) but this cohort may have differed from symptomatic women who have been the common target of such studies. The only statistically significant factor in the Koss study was delayed onset of menopause (see Table 13-8). The full extent of the clinical epidemiology of the disease is deserving of further studies comparing symptomatic with asymptomatic patients.
Clinical Symptoms: Application of Cytologic Techniques
The principal clinical symptom associated with endometrial carcinoma is abnormal bleeding. Endometrial carcinoma is rare in women below the age of forty. Any woman 40 years of age or older who shows clinical evidence of abnormal uterine bleeding for which no obvious cause can be found by obstetrical history or on clinical examination, should be, a priori, suspected of harboring endometrial cancer. A diagnostic workup, at least an endometrial biopsy, but preferably an endometrial curettage, should be obtained without delay. Cytology should not be used as a diagnostic weapon in obvious clinical situations unless a curettage cannot be performed. However, endometrial cancers may produce no symptoms whatever or only insignificant symptoms (such as discharge or spotting) that are not readily elicited on routine questioning of the patient. Such lesions may be discovered by cytologic techniques, and their diagnosis constitutes the chief application of cytology to the detection of endometrial cancer.
CLASSIFICATION OF ENDOMETRIAL CARCINOMAS AND THEIR PRECURSORS
It is generally assumed that endometrial carcinoma is preceded by a series of molecular-genetic and morphologic modification of structure and configuration of endometrial epithelium and glands. Two pathways of disease have been advocated (Sherman, 2000). For the common endometrioid type of endometrial carcinoma, the precursor lesion is known as endometrial hyperplasia. For the relatively uncommon serous carcinoma, the precursor lesion has been named intraepithelial carcinoma.
Histologic make-up of endometrial cancer may have considerable bearing on cytologic diagnosis because tumors of high grade with marked nuclear abnormalities are much easier to recognize than very well differentiated low grade tumors with relatively trivial nuclear changes. The classification of endometrial carcinomas and their precursor lesions, modified from the WHO classification (Scully et al, 1994), is shown below.
Endometrioid carcinoma
Villoglandular carcinoma
Endometrioid carcinoma with squamous differentiation (adenoacanthoma, adenosquamous carcinoma)
Squamous carcinoma
Precursor lesions of endometrioid carcinoma-endometrial hyperplasia
Simple proliferative hyperplasia
Atypical hyperplasia, carcinoma in situ (Hertig)
Serous (papillary serous) carcinoma
Intraepithelial carcinoma
Rare type of carcinomas
Endometrioid Carcinoma
Histology
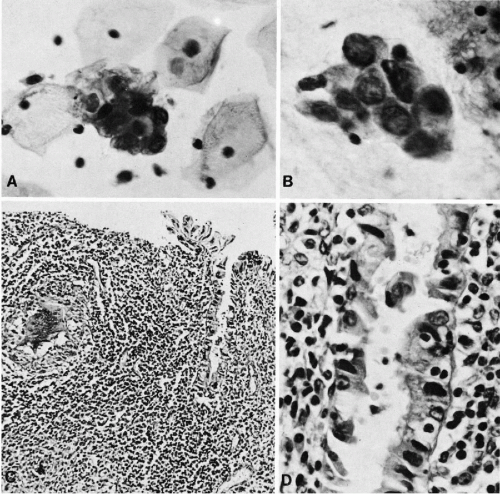
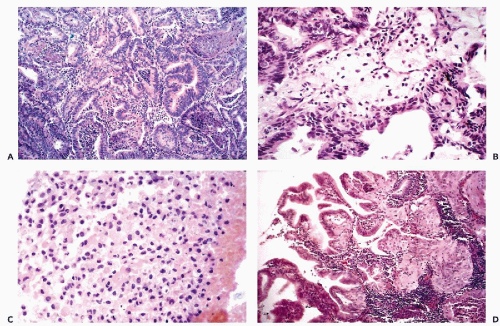
As the name indicates, this malignant tumor is characterized by a disorderly proliferation of the endometrial glands resulting in a grotesque image of the endometrium. These tumors are usually primary in the endometrium but may also develop in endometrial polyps and in foci of endometriosis that may be located in a variety of primary sites, including the ovary and even the regional lymph nodes (Koss, 1963). The cancerous glands vary in size and configuration, are often crowded, and adjacent to each other without intervening endometrial stroma. Papillary projections into the lumen of the glands is not uncommon (Fig. 13-6A). The cancerous glands are lined by cells that are larger than normal, usually cuboidal but sometimes columnar (tall-cell carcinoma) in configuration. The nuclei of these cells vary from simple enlargement and slight hyperchromasia in low grade tumors to markedly enlarged, sometimes hyperchromatic nuclei in high grade tumors. A characteristic feature of cells of endometrioid carcinoma is the presence of clearly visible nucleoli. The number and size of the nucleoli also vary with tumor type, with one or two small nucleoli present in well differentiated tumors, when compared with up to four larger nucleoli in
high grade tumors (Long et al, 1958). The degree of nuclear abnormalities is the basis for nuclear grading that is thought to be of prognostic value. The frequency of mitotic figures varies.
high grade tumors (Long et al, 1958). The degree of nuclear abnormalities is the basis for nuclear grading that is thought to be of prognostic value. The frequency of mitotic figures varies.
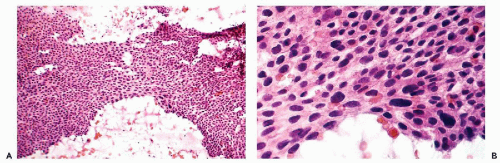
The stroma separating the cancerous glands may occasionally show rather remarkable changes in the form of clusters of very large macrophages, first described by Dubs in 1923 (Fig. 13-6B,C). Rarely, concentric, often calcified protein secretions (psammoma bodies) may be formed by some of these tumors (Parkash and Carcangiu, 1997).
The degree of architectural differentiation of endometrial cancer may vary considerably and is of prognostic significance. Some tumors present only a slight deviation from the normal endometrial pattern (grade I carcinomas, sometimes referred to as adenoma malignum); at the other extreme, there is a grade III carcinoma, presenting as a nearly solid growth of cancer cells in sheets with only an occasional attempt at gland formation. Most of the endometrial cancers fall somewhere between the two extremes and are graded II.
Villoglandular carcinoma is an uncommon variant of endometrioid carcinoma, characterized by formation of slender papillary fronds on the surface of the tumor (see Fig. 13-17B). The tumor cells are similar to those of a well-differentiated endometrioid cancer.
Endometrioid Carcinomas With Squamous Component (Adenoacanthomas and Adenosquamous Carcinomas)
In 25% to 40% of endometrial adenocarcinomas, depending on sampling, a squamous epithelial component may be observed. The histologic appearance of the squamous component may vary from deceptively benign to frankly malignant epidermoid or squamous cancer (Fig. 13-6D). The term adenoacanthoma has now been dismissed but I still find it useful in describing tumors with the histologically benign squamous component. The tumor type with malignant squamous component is usually classified as adenosquamous carcinoma. There is little doubt, however, that, regardless of its degree of differentiation and microscopic appearance, the squamous component in adenoacanthomas is malignant and even capable of metastases. We observed several cases in which the metastatic foci in the lungs were represented solely by the “benign” squamous component. The malignant nature of the squamous component has been confirmed by comparative genomic hybridization studies performed in this laboratory, that documented the presence of chromosomal abnormalities similar to those occurring in cancerous glands (Baloglu et al, 2000).
In fact, in our experience, the occurrence of squamous “metaplasia” in material from endometrial curettings
should always be viewed with suspicion, as it may represent fragments of low-grade adenoacanthoma. There is no known prognostic difference between endometrial adenocarcinomas with or without the squamous component (Marcus, 1961; Pokoly, 1970), although an unfavorable prognosis has been recorded for patients with adenosquamous carcinoma treated by radiotherapy (Ng et al, 1973). Pure squamous cancers of the endometrium may occur, though rarely, and usually in older women (Peris et al, 1958; White et al, 1973; Houissa-Vuong et al, 2002).
should always be viewed with suspicion, as it may represent fragments of low-grade adenoacanthoma. There is no known prognostic difference between endometrial adenocarcinomas with or without the squamous component (Marcus, 1961; Pokoly, 1970), although an unfavorable prognosis has been recorded for patients with adenosquamous carcinoma treated by radiotherapy (Ng et al, 1973). Pure squamous cancers of the endometrium may occur, though rarely, and usually in older women (Peris et al, 1958; White et al, 1973; Houissa-Vuong et al, 2002).
Precursor Lesions of Endometrioid Carcinoma: Endometrial Hyperplasia
It is commonly thought that endometrioid carcinoma is preceded by precursor stages of endometrial carcinoma known as endometrial hyperplasia of various types.
Risk Factors
Hyperplasia, which occurs mainly in premenopausal women, is caused by a hormonal imbalance in favor of estrogens and may result from disturbances of ovulation, such as the Stein-Leventhal syndrome, in which the estrogenic phase is not followed by a progesterone phase. Hormone-producing ovarian tumors, such as theca or granulosa cell tumors, may also produce endometrial hyperplasia. Simple hyperplasia may also be associated with leiomyomas (Deligdisch and Loewenthal, 1970). In postmenopausal women, administration of unopposed exogenous estrogens is a known cause of hyperplasia (the Writing Group for the PEPI Trial, 1996).
Clinical Features
The essential clinical feature of endometrial hyperplasia, regardless of type, is a period of amenorrhea followed by uterine bleeding that may be excessive in amount (menorrhagia) or irregular (metrorrhagia). In some patients, the bleeding may be fairly cyclic in character, whereas in others it is very irregularly spaced.
Histology
Although current textbooks and atlases of gynecologic pathology (e.g., Silverberg and Kurman, 1992) offer a variety of terms to describe various forms of endometrial hyperplasia, according to the configuration of the glands and the level of abnormalities in the epithelial lining, a simple classification is used here. Three forms of endometrial hyperplasia can be distinguished:
Simple proliferative hyperplasia (endometrial hyperplasia with simple tubular glands without nuclear abnormalities)
Cystic hyperplasia, which is probably a variant of simple hyperplasia
Atypical hyperplasia (endometrial hyperplasia with nuclear abnormalities)
This classification disregards the configuration of the glands, but experience has shown that in most hyperplasias with nuclear abnormalities, the endometrial glands are abnormally configured.
Simple Proliferative Hyperplasia
Simple endometrial hyperplasia is an abnormality of endometrial growth in which the equilibrium between the proliferative and the desquamative processes is disturbed in favor of the proliferative phase. In this form of endometrial hyperplasia, the pattern of the endometrium is characterized primarily by an increase in the number of tubular endometrial glands or their cross-sections per low-power field. The glands are separated from each other by endometrial stroma. Often, the glands show slight variability in size and irregular shapes and thus differ from the normal, tubular proliferating glands, which appear round in cross-section (Fig. 13-7A,B). The epithelial cells lining the hyperplastic glands tend to pile up and are often arranged in a somewhat disorderly fashion (loss of polarity). Under high power of an optical microscope and, even more so, by scanning electron microscopy, cilia are commonly observed on the surfaces of the endometrial glandular cells, a feature normally associated with the estrogenic phase of endometrial proliferation (see Chapter 8). Mitotic activity may take place at all levels of the epithelium. The size of the nuclei reflects phases of the cell cycle. Most nuclei are of normal size. Occasionally, however, the nuclei are slightly enlarged, reflecting late phases of cell cycle, and contain small nucleoli, changes that may also be observed in normal endometrium in proliferative phase.
Simple proliferative hyperplasias do not show any chromosomal abnormalities by comparative genetic hybridization and, therefore, must be considered as a benign disorder (Baloglu et al, 2000). These lesions are polyclonal by molecular techniques, whereas malignant lesions are usually monoclonal (Mutter et al, 2000).
Clinical Significance.
In many premenopausal women, the restoration of the ovulatory cycle by hormonal manipulation has resulted in the return to a normal endometrial pattern (the Writing Group for the PEPI Trial, 1996). Return to normal may also be expected after removal of estrogen-producing ovarian tumors. Yet, in rare cases, proliferative hyperplasia of long duration may become associated with atypical hyperplasia and endometrial carcinoma. Whether these are coexisting incidental events, as advocated by Horwitz et al (1981) or reflect some, as yet unknown, common pathway among these lesions, cannot be stated at this time.
Cystic Hyperplasia (Swiss Cheese Hyperplasia)
This disorder is seen mainly in peri- and postmenopausal women, although it may occasionally occur in premenopausal women. The endometrial glands are of variable sizes but most are markedly dilated and cystic. Their lumina are either empty or filled with amorphous material and debris. The epithelial lining of the glands is quite variable and may be separated into active and inactive forms. When the disease is observed in premenopausal women, the gland lining is usually “active” and resembles that
of simple proliferative hyperplasia, described above. In postmenopausal women, the gland lining is “inactive,” consisting of a single layer of cuboidal cells without any evidence of proliferative activity. In the latter situation, the disease must be differentiated from cystic atrophy of the endometrium (see Chap. 8).
of simple proliferative hyperplasia, described above. In postmenopausal women, the gland lining is “inactive,” consisting of a single layer of cuboidal cells without any evidence of proliferative activity. In the latter situation, the disease must be differentiated from cystic atrophy of the endometrium (see Chap. 8).
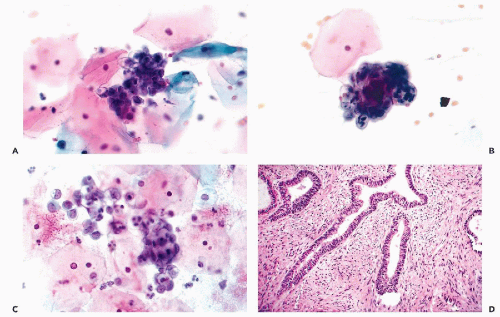
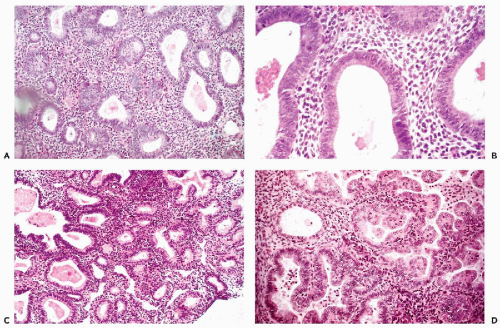 Figure 13-7 Endometrial hyperplasia and Hertig’s carcinoma in situ. A,B. Simple endometrial hyperplasia with cystic dilatation of glands. The epithelium of these glands is often ciliated. C. Complex (atypical) hyperplasia in which the glands are numerous, crowded, and of unequal size and irregular configuration. D. A form of atypical endometrial hyperplasia in which the glands form papillary projections lined by tall cells with eosinophilic cytoplasm. This lesion, named carcinoma in situ, was observed by Hertig et al (1949) in endometrial curettage specimens obtained some years before the development of an endometrioid carcinoma. |
It is likely that cystic hyperplasia represents an end stage of involution of the simple proliferative endometrial hyperplasia. The association of this form of hyperplasia with endometrial adenocarcinoma is uncommon, but I have repeatedly observed such lesions side by side.
Atypical Hyperplasia
Atypical or adenomatous hyperplasia is defined by an increase in the number of endometrial glands of various sizes and variable configuration per low-power field, usually associated with nuclear abnormalities in cells of the glandular epithelium (Fig. 13-7C). The atypical glands are separated from each other by endometrial stroma, although “back to back” glands, without intervening stroma, are also seen.
The epithelial cells in most of these lesions are similar to cancer cells because they are frequently enlarged, have enlarged nuclei with prominent nucleoli, and show intense mitotic activity at all levels of the epithelium. As in endometrioid carcinomas, the stroma may show accumulation of large macrophages.
In an important retrospective study by Hertig et al (1949), the precursors of endometrioid carcinoma were classified as endometrial carcinoma in situ, to be differentiated from the newly established entity, endometrial intraepithelial carcinoma (EIC), the precursor lesion of the serous-papillary carcinoma. Endometrial carcinoma in situ is a form of atypical hyperplasia that was observed in prior endometrial biopsies and curettage material in women who subsequently developed endometrioid carcinomas. This lesion was characterized by endometrial glands of variable, irregular configuration, lined with large, usually columnar cells with eosinophilic cytoplasm, forming either single or multiple layers. Papillary proliferation and bridging of the lumen of the gland by proliferating epithelial cells may be observed. The nuclei, which occupy variable positions in relation to the lumen, are enlarged, vesicular, and usually contain visible nucleoli. The degree of cell abnormality is better appreciated if the gland lumen contains desquamated cells; these often show nuclear hyperchromasia and large nucleoli (Fig. 13-7D).
Comparative genomic hybridization disclosed that the atypical hyperplasia, even with trivial nuclear abnormalities, shares with endometrioid carcinoma a number of chromosomal abnormalities and, therefore, should be considered a precancerous lesion or an early stage of endometrioid carcinoma (Baloglu et al, 2000). It is not surprising, therefore, that in many instances the histologic differentiation of atypical hyperplasia from early carcinoma is a matter of dispute among competent pathologists. In fact, photographs of the two lesions in various publications could often be substituted for one another. One could repeat verbatim the statement regarding the differential diagnosis of precancerous lesions of the cervix, that “every debatable case could become a ‘shopping slide,’” ultimately handled by ablation of the uterus, not out of knowledge, but out of desperation. The famous saying “kein Karzinom aber besser heraus” (not a carcinoma but better take it out), attributable to a German gynecologist, Halban (cited by Novak, 1956), pertains to atypical hyperplasia. Some observers proposed the term endometrial intraepithelial neoplasia (EIM), to encompass atypical hyperplasia and well differentiated endometrioid carcinomas (Sherman and Brown, 1979; Fox and Buckley, 1982), a term that reflects the realities of the situation. The term has been revived recently by an Endometrial Collaborative Group that included 19 gynecologic pathologists from several countries by adding molecular biologic criteria (Mutter et al, 2000). Monoclonality and instability of microsatellites, were the principal molecular abnormalities linking EIM to endometrial carcinoma.
The relationship of simple proliferative hyperplasia to atypical hyperplasia is not clear and one cannot rule out the possibility that the benign form may sometimes be transformed into the malignant form.
The differential diagnosis of endometrial hyperplasia in curetted material includes endometrial polyps, artifacts produced by dull curettes, secretory endometrium in the premenstrual stage showing see-saw appearance of endometrial glands, and the glands of the endometrial basal layer, which are often somewhat dilated and irregular in shape.
Role of Hyperplasia in the Genesis of Endometrial Carcinoma
Evidence for progression of atypical hyperplasia to carcinoma of the endometrium is relatively poor because most of these lesions cause symptoms and are treated, at least by curettage and hormonal manipulation, but not infrequently by hysterectomy. At the time of this writing (2004), few patients with these abnormalities are left untreated. The evidence of progression is based on older studies. A frequently cited study is that by Gusberg and Kaplan (1963) in which a group of patients with “adenomatous hyperplasia” were prospectively followed; several of them (about 10%) developed endometrial cancer. Anecdotal evidence of progression of endometrial hyperplasia to carcinoma was also provided by Foster and Montgomery (1965). In a retrospective study of 170 patients, Kurman et al (1985) classified hyperplasias according to the degree of nuclear abnormality. Carcinoma developed in only 2 of 122 patients without significant cytologic atypia and in 11 of 48 women (23%) with “atypical” glands. The “progression” also depended on the complexity of the glandular pattern with “simple” lesions less likely to progress than “complex” lesions. Many of the lesions illustrated in the Kurman paper as “atypical complex hyperplasia” could be classified by other observers as a well-differentiated endometrioid carcinoma. Further, even though none of these patients were initially treated by hysterectomy, most received some form of treatment such as hormonal manipulation, curettage, or both. Hence, the rate of development of invasive cancer in untreated patients could be much higher.
However, there is substantial evidence suggesting that endometrial hyperplasia is not a mandatory stage in the development of endometrioid carcinoma (or other types of endometrial cancer) that may also develop de novo, particularly in postmenopausal women. The search for occult endometrial cancer (Koss et al, 1981, 1984) strongly suggested this possibility (see below). In an older contribution, Greene et al (1959) observed peripheral hyperplasia in only 10 of 120 cases of endometrial carcinomas. These authors expressed the view that, “some (and probably the minority) of endometrial carcinomas are preceded by or possibly induced in or developed from areas of endometrial hyperplasia.” These observations are particularly valuable because they were published in 1959, before widespread use of hormones obscured endometrial pathology.
Based on a case control study, cited above, Horwitz and Feinstein (1978) proposed that “endometrial hyperplasia and carcinoma may represent separate expressions of endometrial pathology, which may occur side by side, but do not necessarily follow each other. It is further suggested that the so-called atypical hyperplasia, a lesion most likely to ‘progress’ to invasive carcinoma, does in fact represent a low-grade endometrial carcinoma. The two lesions can only be separated from each other by a series of intricate and generally nonreproducible morphologic criteria.” Still, endometrial hyperplasia of whatever type must be construed as a warning sign that an endometrium is not cycling or not cycling properly and, therefore, is susceptible to neoplastic events. With luck and skill, the cytologic diagnosis of occult endometrial hyperplasia is sometimes possible either in cervicovaginal smears or in direct endometrial samples.
It has been reported that hormonal manipulation of atypical hyperplasia with progesterone and related drugs may occasionally restore the cycling endometrial pattern (the Writing Group for the PEPI Trial, 1996). Yet, in our experience, these drugs are rarely, if ever, curative of the disease. There is little doubt, however, that the presence of these abnormalities puts the untreated patient at risk for the development of endometrial carcinoma, although the degree of risk cannot be estimated in any individual patient.
Serous (Papillary Serous) Carcinoma
About 10% of endometrial cancers that are similar to ovarian tumors of comparable configuration have been recognized
many years ago as tumors with poor prognosis, capable of forming metastases, even if diagnosed in early stages (Chen et al, 1985). The tumors are composed of large malignant cells, often forming papillary structures that may contain psammoma bodies (Spjut et al, 1964; Factor, 1974). It must be stressed, however, that psammoma bodies may also occur in endometrioid carcinoma, in benign endometria, and endometrial polyps in the absence of cancer. Quite often, the tumors infiltrate the myometrium as poorly formed glands or solid strands of tumor cells. Mutation of p53 gene occurs in the primary tumor and its metastases (Baergen et al, 2001).
many years ago as tumors with poor prognosis, capable of forming metastases, even if diagnosed in early stages (Chen et al, 1985). The tumors are composed of large malignant cells, often forming papillary structures that may contain psammoma bodies (Spjut et al, 1964; Factor, 1974). It must be stressed, however, that psammoma bodies may also occur in endometrioid carcinoma, in benign endometria, and endometrial polyps in the absence of cancer. Quite often, the tumors infiltrate the myometrium as poorly formed glands or solid strands of tumor cells. Mutation of p53 gene occurs in the primary tumor and its metastases (Baergen et al, 2001).
Precursor Lesions of Serous Carcinoma
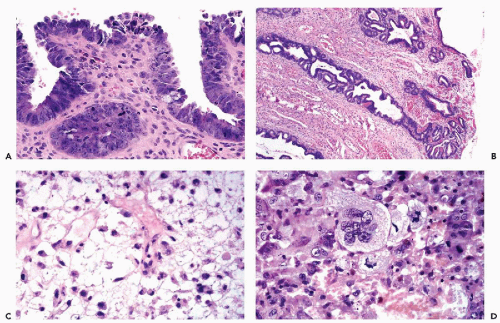
Recent studies of this group of tumors traced their origin to malignant changes in the surface endometrium and adjacent glands that has been labeled endometrial intraepithelial carcinoma (Fig.13-8A,B), and which is characterized by expression of mutated protein p53 (Sherman et al, 1992, 1995, 2000). On the surface, the lesion is composed of a single or double layer of large cancer cells with large nuclei and nucleoli, sometimes in a palisade arrangement. Adjacent glands show similar changes. Mitotic activity is abundant. The proponents of EIC avoided the use of the term endometrial carcinoma in situ, an abnormality of endometrial glands, described by Hertig et al (1949) as a precursor lesion of endometrioid carcinoma, discussed above. It has been proposed that the genesis of serous endometrial carcinoma follows a different pathway from endometrioid carcinoma and is unrelated to endometrial hyperplasia (Sherman et al, 1992, 1995, 2000).
Rare Histologic Variants of Endometrial Carcinoma
Endometrial carcinomas may show evidence of secretory activity (secretory carcinomas) that may be a mucin-like substance (mucinous carcinomas). Such tumors should be differentiated from endocervical carcinoma. Some endometrial tumors are composed of “clear” cells, i.e., cells with transparent cytoplasm, showing cell arrangement not unlike that seen in similar tumors of the uterine cervix and vagina (clear cell carcinomas; Fig. 13-8C). Other rare types of endometrial cancer include carcinomas with argyrophilic cells (Ueda et al, 1979; Aguirre et al, 1984), small cell (oat cell) type (Paz et al, 1984), carcinoma with “glassy cell features” (Arends et al, 1984), carcinoma with ciliated cells (Hendrickson and Kempson, 1983; Gould et al, 1986; Maksem, 1997) and carcinoma with giant cells, resembling osteoclasts (Fig. 13-8D) (Jones et al, 1991).
Occasionally, endometrial carcinomas are composed in part of spindly malignant cells (spindle cell carcinomas or carcinosarcomas). The differential diagnosis of these tumors with mesodermal mixed tumors is discussed in Chapter 17.
Staging and Prognosis
Endometrial carcinoma is staged according to the spread of the disease. In stage I, the disease is confined to the corpus, subdivided into Ia (depth of uterine canal less than 8 cm) and Ib (depth of uterine canal 8 cm or more). Stage II disease indicates involvement of corpus and cervix. Stage III indicates extension beyond the uterus but still confined within the bony pelvis, and stage IV indicates spread to the bladder and/or rectum, or evidence of distant metastases. Tambouret et al (2003) pointed out that extension of endometrial carcinoma to the uterine cervix may have a deceptively benign appearance in histologic sections. The role of peritoneal washings in staging of endometrial cancer is discussed in Chapter 16. Staging may also include histologic grade (G) of the lesion, discussed above, with G1 indicating a well-differentiated carcinoma, G3 poorly differentiated cancer, and G2 cancer of an intermediate grade. Poor prognosis of serous carcinoma, regardless of stage, has been mentioned above.
The results of treatment are by no means spectacular; only stage I G1 lesions respond well and offer a nearly 100% 5-year cure. For all stages and grades, the 5-year survival rate is only about 65%, and this figure has not changed much over the years (Frick et al, 1973; Prem et al, 1979; Robboy and Bradley, 1979; Partridge et al, 1996). More recent figures, based on a very large cohort of women in Norway, reported 5-year survival for all stages at 78% and 10-year survival at 67% (Abeler et al, 1992). The survival was stage dependent, with best results reported for stage I disease, and the poorest for stage IV. Hence, endometrial carcinoma is a serious, often misunderstood, disease and its early detection is a worthwhile undertaking.
Other Features of Prognostic Significance
Tumor Ploidy
DNA ploidy measurements have been shown to be of prognostic value in endometrial carcinoma (Atkin, 1984; Iverson and Laerum, 1985; Iverson and Utaaker, 1988; and others). It has been documented that tumors with approximately diploid DNA content have a better prognosis than aneuploid tumors. In general, well-differentiated endometrioid carcinomas have a diploid DNA content but occasionally higher grade tumors are also in the diploid range of measurements.
Morphometric Studies
Baak et al (1988) reported that combined architectural and nuclear morphometric features in tissue sections were a more accurate predictor of behavior of endometrial hyperplasia than nuclear features alone. This elaborate study requiring costly instrumentation and dedicated personnel is not likely to be of practical value in the laboratory.
Steroid Receptors
These studies have documented the presence of estrogen and progesterone receptors in most endometrial carcinomas and in some metastases (Ehrlich et al, 1981




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree