Appendix The processing of lung specimens
Whilst the value of cytopathology is fully acknowledged,1–3 this book is concerned with histopathology and except for a section on bronchoalveolar lavage, consideration will be largely limited to specimens submitted for histology. Lung specimens are obtained by a variety of procedures and vary greatly in size (Box A.1). It is desirable that all specimens be received in the laboratory unfixed but this is best waived in the case of small biopsies because of their greater risk of drying in transit. For these specimens the clinician should be provided with small containers of fixative; for larger specimens dry containers should be provided with instructions that they should be brought to the laboratory straight away. A refrigerator should be available for specimens brought to the laboratory out of hours. The various types of specimen will be dealt with in turn but consideration must first be given to laboratory safety.
Safe A handling of lung specimens
It is incumbent upon the clinician to inform the pathologist if there is any possibility of the specimen being infectious but this cannot always be relied upon and it is good practice to regard all fresh tissue as being potentially infectious. To minimise the risk of laboratory infection the tissue should be immersed in formaldehyde, ideally for 72 hours before it is examined,4 but this is not generally practicable. Not infrequently, the clinical situation demands a frozen section. Then the selection of tissue should take place in a microbiological safety cabinet and afterwards the cryostat cabinet should be fumigated with formaldehyde vapour overnight. Other specimens also need to be first handled in a microbiological safety cabinet, either to immerse or fill them with formaldehyde. By the next day most microbes will no longer be viable but tubercle bacilli and prions can survive much longer and continued caution is necessary.
Formaldehyde is categorised as a probable carcinogen by the International Agency for Research on Cancer.5 Several governments have therefore stipulated maximum airborne levels in the workplace. These levels are easily exceeded when copious amounts are used to distend lungs unless an adequately ventilated bench or cabinet is used.
Needle aspiration
Needle aspiration is appropriate for solid lesions such as tumours whereas pulmonary infiltrates seldom produce satisfactory specimens if sampled by this technique. Whatever the site of the lesion within the thorax, special sampling procedures are required, namely bronchoscopy for transbronchial aspiration, oesophagoscopy for transoesophageal aspiration and radiography for transcutaneous aspiration. The pathologist is therefore seldom involved in the actual sampling procedure, which is generally undertaken by a clinician or a radiologist. Ultrasound guidance has greatly improved the accuracy of transbronchial and transoesophageal needle aspiration, which are being increasingly used for the diagnosis of diseases affecting the mediastinal lymph nodes, such as stage 1 sarcoidosis, and in the assessment of mediastinal lymph node status when staging lung cancer.6–10
The usual fine needles (22-gauge) produce a cell suspension that is suitable for cytology but it is increasingly the case that extensive immunocytochemistry and gene mutational analysis are required in addition to a microscopical diagnosis. These can all be achieved if the aspirate is centrifuged to produce a cell pellet, which may be sectioned, or submitted intact for gene analysis (see below).10,11 Alternatively, the slightly wider 18- or 19-gauge needles may be used. These produce a thin core of tissue that can be processed for histology in the conventional way,12,13 especially if the transcutaneous needle is inserted with the aid of a spring-loaded firing device.14–16 With this technique the aspirated material (which may be contained in the needle as much as the syringe) can be rinsed directly into the fixative as soon as practicable and processed in the laboratory in the same way as small biopsies. The principal complications are a relatively small pneumothorax and haemoptysis, encountered in 23% and 4% of patients respectively at one institution, with no fatalities.17 As with any invasive technique the danger of tumour seeding in the needle track has to be kept in mind.18,19
Bronchial and transbronchial biopsy
The rigid bronchoscope is nowadays largely confined to therapeutic procedures such as stemming haemorrhage, the extraction of a foreign body, the insertion of a stent and laser ablation. The flexible fibreoptic bronchoscope is generally preferred for diagnostic use. It reaches further and can enter upper-lobe airways (Fig. A.1). The specimens are smaller but several may be taken.
Bronchoscopic biopsies may contain only bronchus, only pulmonary parenchyma or both. If the specimen floats it is more likely to contain alveoli than not but no less likely to be diagnostic or abnormal than if it sinks.20,21 A note of the number of fragments received provides a useful check on laboratory procedures but if this information is incorporated in the report it may be disputed by bronchoscopists who have made their own count. Discrepancies are often due to the clinician counting flecks of mucus as tissue fragments. Reporting the number of fragments received is therefore not conducive to good professional relationships. However, it is imperative that no fragment be lost in processing and to minimise this risk it is advisable that the fragments are handled just once, if at all. It is advantageous if the clinician wipes the specimens on to a small strip of filter paper and puts this into the specimen jar. Alternatively, if loose fragments are received they should be transferred with fine-toothed forceps into a capture vehicle such as tissue paper, a fine-mesh wire cage or molten agar, which when set can be further processed as if it were a button of tissue. The agar survives processing and can seen around the tissue when the slide is examined by the naked eye but is invisible when the section is examined microscopically.22 Imprisoning the fragments in sponge is likely to cause tissue artefacts.23 Free tumour cells may remain in the fixative or other transport medium after all fragments have been removed and the sensitivity of the bronchoscopy procedure is marginally improved if the residual fluid is submitted for cytology.24
The largest fragments of lung obtained by transbronchial biopsy each contain about 100 alveoli. If the changes are non-specific and few alveoli have been obtained, the paucity of lung tissue should be made clear in the report. At least three step sections should be prepared, keeping spares at each level for any special stains that might be required.25 If no abnormalities are identified at any of these levels and tissue remains in the block, further step sections should be prepared. Many laboratories, particularly those dealing with specimens from lung transplant or otherwise immunocompromised patients, routinely examine step sections, or even serial sections through the block. In immunodeficient patients it is advisable to stain for fungi and mycobacteria routinely as the histopathological changes may be atypical.26
Several diagnostic pitfalls are inherent in the interpretation of fibreoptic biopsies:
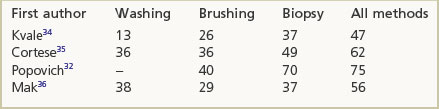
When a tumour is visible to the bronchoscopist the sensitivity of a forceps biopsy ranges between 70% and 100%.28–31 When the tumour is not visible the diagnostic sensitivity is less and varies with the location and size of the lesion. The yield is increased if multiple specimens are obtained. One study found that with central tumours a single biopsy succeeded in establishing the diagnosis in 92% of cases, a figure that increased to 96% if four samples were taken, but was not further improved upon with six. For peripheral tumours, positive results increased progressively from 45% through 55% and 60% to 75% when one, four, five and six samples respectively were taken.32 Sputum examination is probably not cost-effective except when the patient has a tumour that is evidently unresectable.33 However, cytology is appropriate for the evaluation of fine-needle transbronchial needle aspirates of mediastinal lymph nodes, which contributes to the staging of lung cancer. In some circumstances cytology and histology augment each other (Box A.2).32,34–36
In many of the diffuse parenchymal lung diseases a transbronchial biopsy is often uninformative, but it may provide an unexpected tissue diagnosis, obviating the need for more invasive procedures. Thus, in a patient suspected of having idiopathic pulmonary fibrosis, a disease in which fibreoptic specimens are seldom diagnostic, a bronchoscopic biopsy may identify unsuspected sarcoidosis and so circumvent the necessity for a surgical procedure. Indeed, this condition is often recognisable histologically even if an attempted transbronchial procedure samples only the bronchus. This is because the bronchus has a rich lymphatic supply and sarcoidosis has a lymphangitic distribution. The likelihood of transbronchial biopsy giving a diagnosis in various lung diseases is shown in Box A.3.
Box A.3 Fibreoptic biopsy
utility in various lung diseases
| Examples | |
|---|---|
| Excellent for diffuse bronchial or lung disease | Sarcoidosis |
| ‘Lymphangitis carcinomatosa’ | |
| Systemic amyloidosis | |
| Good for mass lesions or patchy disease that can be targeted | Tumours |
| Pneumocystis pneumonia | |
| Eosinophilic pneumonia | |
| Alveolar lipoproteinosis | |
| May help in patchy disease that cannot be targeted | Langerhans cell histiocytosis |
| Poor in diseases with scanty specific features or those in which study of the lobular architecture is important to the diagnosis | Extrinsic allergic alveolitis |
| Interstitial pneumonias (idiopathic pulmonary fibrosis) |
The diseases unlikely to be identified in these small specimens are those such as idiopathic pulmonary fibrosis and extrinsic allergic alveolitis in which diagnosis depends upon their lobular distribution.37–39 The number of fibreoptic specimens required to give diagnostic material has been studied in regard to sarcoidosis. One group found that a single biopsy provided a tissue diagnosis of sarcoidosis in 46% of cases, increasing progressively to 90% when four biopsies were taken but not appreciably increasing further thereafter.40 On comparing the diagnostic yield in patients with pulmonary infiltrates a diagnosis is reported to be provided by transbronchial biopsy in 59% and by open-lung biopsy in 94%.41 In the diagnosis of cryptogenic organising pneumonia, transbronchial biopsy is reported to have a sensitivity of 64% and a specificity of 86%.42
Open and video-assisted thoracoscopic lung biopsies
From the pathologist’s point of view there is little difference between a biopsy obtained at thoracotomy (open biopsy)43,44 and one obtained at video-assisted thoracoscopy.45–48 Both give good-sized specimens and both afford the surgeon a choice of biopsy site. The optimum biopsy site often depends upon the nature of the disease being investigated. Nodules are best excised in their entirety or if this is impracticable a wedge should be taken to include both the core of the lesion and its interface with its surroundings. Diffuse lung disease often shows a zonal difference in disease activity and it is therefore advantageous if both the upper and lower lobes are sampled. Alternatively, a site thought to show intermediate changes may be chosen. Some advise that the tip of the lingula, or indeed the tip of any lobe, should be avoided as these sites often show non-specific scarring,49 but this is disputed.50,51 As noted above the biopsy procedure often gives rise to alveolar haemorrhage and care is needed in the interpretation of blood in the air spaces. It is likely to be genuine if there is also haemosiderosis.
In many cases the lung sample will have collapsed on removal and, if fixed in this state, there will be close apposition of the alveolar walls. This makes it difficult to study the architecture of the lung and can lead to considerable misinterpretation. The lung can however be reinflated by vacuum52 or better by injection, the latter achieved by gently instilling a little fixative into the specimen with a syringe, taking care not to overexpand the tissue.53 Airways cannot easily be identified by eye in these peripheral lung specimens and it is sufficient to inject the fixative directly into the lung parenchyma. The only disadvantage of this procedure is that free cells may be cleared from consolidated alveoli and diagnoses such as desquamative interstitial pneumonia obscured. However, the benefits of gentle inflation–fixation outweigh this single disadvantage.
Electron microscopy requires very little tissue but has its own requirements, the most important of which is prompt fixation. Whereas light microscopy is often satisfactory despite delayed fixation, this is not true of electron microscopy. If the recommended fixative (glutaraldehyde or methanol-free formaldehyde) is not available, commercial formalin is an acceptable alternative so long as fixation is rapid. Archival tissue rescued from old paraffin blocks is often satisfactory for electron microscopy if it had been fixed without delay. Rapid fixation for electron microscopy is also possible postmortem by injecting fixative through the chest wall immediately after death; a marker dye in the fixative facilitates recognition of the injected site when autopsy is undertaken.54 Special care should be taken against ‘crush’ artefact when selecting small tissue samples for electron microscopy; a new razor blade should be used for each specimen and cutting effected with a to-and-fro motion rather than by exerting downwards pressure.
Pleural biopsy
The pleura has traditionally been sampled blind using a reverse-bevel needle such as the Abrams or Cope. The sensitivity rises with the number of specimens obtained, four to six being the optimum.55,56 Some report that at best blind biopsy has a sensitivity of 40–50%,56 which may be improved by the use of image guidance by computed tomography or ultrasound to about 90%57 but for mesothelioma others report that blind biopsy has a sensitivity of only 16% and therefore recommend sampling by open or video-assisted thoracoscopy, when the sensitivity rises to 100%.58 If fluid is found opinions vary with regard to the volume there should be sent for assessment of malignancy, but 50–60 ml is a reasonable compromise.59,60
Frozen section
Most frozen sections of lung tissue are performed for the evaluation of solid lesions, the assessment of tumour margins or the detection of mediastinal lymph node metastasis.61 It is best avoided on lung tissue that is not consolidated but if it is necessary the tissue may first be inflated with frozen section mounting medium. Intraoperative assessment of mediastinal lymph node status by frozen section has diminished with the improvement in preoperative assessment by ultrasound-guided fine-needle. If a tumour of uncertain origin is identified by frozen section, immunohistochemistry is feasible for distinguishing primary from secondary lung tumours in this setting.62
Whole lungs and lung lobes
Other procedures that might be considered before either fixation or cut-up include injection of blood vessels or airways, taking care to remove as much clot or bronchial secretions as possible and afterwards excluding these as possible causes of any blockages or apparent stenoses. A coloured or radiopaque gelatine mixture (e.g. Micropaque barium sulphate suspension 330 ml, gelatine 33 g, water 130 ml) containing thymol as a preservative may be stored for this purpose, melting the solid mix as required by immersion in warm water, but not heating it above 56°C as this would denature the gelatine (see Figs 1.35 and 3.14B, pp. 21 and 104).63,64
Radiography is also possible after fixation if the lungs are inflated with formaldehyde fume65 or if lung slices are air-dried after being fixed with liquid formaldehyde.66–68 Air-drying is facilitated if the fixative comprises 10 parts polyethylene glycol 400, 5 parts ethanol, 2 parts formalin and 3 parts water. However, both these procedures have been rendered largely redundant by preoperative contrast-enhanced studies that generally provide far superior images.
The choice of cut-up procedure is wide and depends upon the nature of the underlying disease. Different procedures are appropriate for studying a widespread process such as emphysema than for a localised one such as a tumour. In other circumstances it may be desirable to open the airways or the blood vessels widely, or possibly both without cutting across either. This can be achieved if the airways are opened from the hilum and the arteries from the oblique fissure.69
Corrosion casts of pulmonary blood vessels or airways constitute another way of demonstrating abnormalities in the lung,70 albeit at the cost of losing all intervening tissue (see Figs 1.3 and 1.10, pp. 4 and 8). However, bronchial cartilage can be studied without losing the intervening tissue by staining the cartilage with toluidine blue and clearing the specimen with an organic solvent such as cedarwood oil or xylol.71
Diffuse lung disease
To demonstrate emphysema, or other diffuse disease, sagittal slicing is customary, and for this a particularly good result can be obtained with an electric meat-slicer. Conditions such as emphysema and fibrosis can then be demonstrated more convincingly with the barium sulphate technique.72 This requires the lung slice to be squeezed gently in a 7.5% solution of barium chloride and then for a 10% solution of sodium sulphate to be poured on, the resultant white precipitate of barium sulphate emphasising structural differences in the lung substance (see Figs 3.14B, 3.15B, 3.16B and 7.1.23, pp. 104–106 and 347). Similar contrast can be achieved by fixing the lung in Bouin’s solution.73 Paper-mounted whole-lung sections74,75 provide an excellent permanent demonstration of conditions such as emphysema (see Figs 1.7, 3.14A, 3.15A, 3.16B, 7.1.9A, 7.1.10A, 7.1.15 and 7.1.16, pp. 7, 104, 105, 106, 334, 335, 340) but an easy alternative is to bag or laminate in plastic the thinnest slices obtainable with a meat-slicer.76
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree