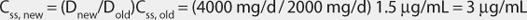Figure 8-1 Procainamide serum concentrations initially drop rapidly after an intravenous bolus as drug distributes from blood into the tissues during the distribution phase. During the distribution phase, drug leaves the blood due to tissue distribution and elimination. After 20-30 minutes, an equilibrium is established between the blood and tissues, and serum concentrations drop more slowly since elimination is the primary process removing drug from the blood. A two-compartment model describes this type of serum concentration/time profile.
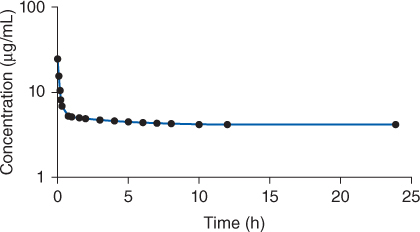
Figure 8-2 To maintain therapeutic procainamide concentrations, an intravenous loading dose (over 25-30 minutes) of procainamide is followed by a continuous intravenous infusion of the drug. A distribution phase is still seen due to the administration of the loading dose. Note that the administration of a loading dose may not establish steady-state conditions immediately, and the infusion needs to run 3-5 half-lives until steady-state concentrations are attained.
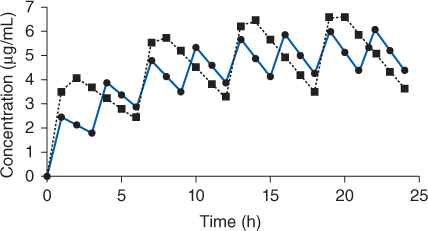
Figure 8-3 Serum concentration-time profile for rapid-release procainamide (solid line, given every 3 hours) or sustained-release procainamide (dashed line, given every 6 hours) oral dosage forms after multiple doses until steady-state is achieved. The curves shown would be typical for an adult with normal renal and hepatic function.
The generally accepted therapeutic range for procainamide is 4-10 μg/mL. Serum concentrations in the upper end of the therapeutic range (≥8 μg/mL) may result in minor side effects such as gastrointestinal disturbances (anorexia, nausea, vomiting, diarrhea), weakness, malaise, decreased mean arterial pressure (<20%), and a 10%-30% prolongation of electrocardiogram intervals (PR and QT intervals, QRS complex). Procainamide serum concentrations above 12 μg/mL can cause increased PR interval, QT interval, or QRS-complex widening (>30%) on the electrocardiogram, heart block, ventricular conduction disturbances, new ventricular arrhythmias, or cardiac arrest. Procainamide therapy is also associated with torsade de pointes.1,2 Torsade de pointes (twisting of the points) is a form of polymorphic ventricular tachycardia preceded by QT-interval prolongation. It is characterized by polymorphic QRS complexes that change in amplitude and length giving the appearance of oscillations around the electrocardiographic baseline. Torsade de pointes can develop into multiple episodes of nonsustained polymorphic ventricular tachycardia, syncope, ventricular fibrillation, or sudden cardiac death.
Nondose or concentration-related side effects to procainamide include rash, agranulocytosis, and a systemic lupus-like syndrome. Symptoms of the lupus-like syndrome include rash, photosensitivity, arthralgias, pleuritis or pericarditis, hemolytic anemia or leukopenia, and a positive antinuclear antibody (ANA) test. Patients who metabolize the drug more rapidly via N-acetyltransferase II, known as “rapid acetylators,” appear to have a lower incidence of this adverse effect or at least take more time and higher doses for it to appear. While the lupus-like syndrome is usually not life threatening, it does occur in 30% to 50% of patients taking procainamide for greater than 6-12 months and requires discontinuation of the drug. Most symptoms abate within several weeks to months, but some patients have required a year or more to completely recover. Intravenous procainamide doses must be given no greater than 25-50 mg/min, as faster injection can cause profound hypotension.
An active procainamide metabolite, known as N-acetylprocainamide (NAPA) or acecainide, also possesses antiarrhythmic effects.12–14 Based on limited clinical trials of NAPA, effective concentrations are 10-30 μg/mL. Concentration-dependent adverse effects for NAPA are similar to those given for procainamide. However, NAPA does not appear to cause a systemic lupus-like syndrome. Currently, NAPA is not commercially available in the United States and has been given orphan drug status by the U.S. Food and Drug Administration with an indication for decreasing implantable defibrillator energy requirements.15 Some laboratories report the sum of procainamide and NAPA concentrations for a patient as the “total procainamide concentration” using the therapeutic range of 10-30 μg/mL. However, because procainamide and NAPA have different antiarrhythmic potency, serum concentrations for each agent should be considered individually. Also, many individuals feel that it is more important to maintain therapeutic procainamide concentrations in patients rather than NAPA or total procainamide levels in the suggested ranges. Clinicians should understand that all patients with “toxic” procainamide or NAPA serum concentrations in the listed ranges will not exhibit signs or symptoms of procainamide toxicity. Rather, procainamide and/or NAPA concentrations in the given ranges increase the likelihood that an adverse effect will occur.
For dose adjustment purposes, procainamide serum concentrations during oral administration are best measured as a predose or trough level at steady-state after the patient has received a consistent dosage regimen for 3-5 drug half-lives. If the drug is given as a continuous intravenous infusion, procainamide serum concentrations could be measured at steady-state after the patient has received a consistent infusion rate for 3-5 drug half-lives. Procainamide half-life varies from 2.5 to 5 hours in normal adults to 14 hours or more in adult patients with renal failure. Average NAPA half-lives are 6 hours for normal adults and 41 hours for adult patients with renal failure. If procainamide is given orally or intravenously on a stable schedule, steady-state serum concentrations for parent drug and metabolite will be achieved in about 1 day (5 • 5 h = 25 h for procainamide and 5 • 6 h = 30 h for NAPA). For a patient in renal failure, it will take 3 days for steady-state concentrations to occur for procainamide and 9 days for steady-state conditions to be established for NAPA (5 • 14 h = 70 h or ~3 days for procainamide, 5 • 41 h = 205 h or ~9 days for NAPA).
CLINICAL-MONITORING PARAMETERS
The electrocardiogram (ECG or EKG) should be monitored to determine the response to procainamide. The goal of therapy is suppression of arrhythmias and avoidance of adverse drug reactions. Electrophysiologic studies using programmed stimulation to replicate the ventricular arrhythmia or 24-hour ECG monitoring using a Holter monitor can be performed in patients while receiving a variety of antiarrhythmic agents to determine effective antiarrhythmic drug therapy.16
Because many procainamide therapeutic and side effects are not correlated with its serum concentration, it is often not necessary to obtain serum procainamide concentrations in patients receiving appropriate doses who currently have no arrhythmia or adverse drug effects. However, procainamide serum concentrations should be obtained in patients who have a recurrence of tachyarrhythmias, are experiencing possible procainamide side effects, or are receiving procainamide doses not consistent with disease states and conditions known to alter procainamide pharmacokinetics (see Effects of Disease States and Conditions on Procainamide Pharmacokinetics and Dosing section). Serum concentration monitoring can aid in the decision to increase or decrease the procainamide dose. For instance, if an arrhythmia reappears and the procainamide serum concentration is <10 μg/mL, increasing the procainamide dose is a therapeutic option. However, if the procainamide serum concentration is over 10-12 μg/mL, it is less likely a dosage increase will be effective in suppressing the arrhythmia and there is an increased likelihood that drug side effects may occur. Some patients have responded to procainamide serum concentrations as high as 20 μg/mL without experiencing severe adverse effects.17 Similarly, if a possible concentration-related procainamide adverse drug reaction is noted in a patient and the procainamide serum concentration is <4 μg/mL, it is possible that the observed problem may not be due to procainamide treatment and other sources can be investigated. While receiving procainamide, patients should be monitored for the following adverse drug effects: anorexia, nausea, vomiting, diarrhea, weakness, malaise, decreased blood pressure, electrocardiogram changes (increased PR interval, QT interval, or QRS complex widening >30%), heart block, ventricular conduction disturbances, new ventricular arrhythmias, rash, agranulocytosis, and the systemic lupus-like syndrome.
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
Procainamide is eliminated by both hepatic metabolism (~50%) and renal elimination of unchanged drug (~50%).12–14,18,19 Hepatic metabolism is mainly via N-acetyltransferase II (NAT-II).12–14 N-acetylprocainamide or NAPA is the primary active metabolite resulting from procainamide metabolism by N-acetyltransferase II. N-acetyltransferase II exhibits a bimodal genetic polymorphism that results in “slow acetylator” and “rapid acetylator” phenotypes. If the patient has normal renal function, acetylator status can be estimated using the ratio of NAPA and procainamide (PA) steady-state concentrations: acetylator ratio = NAPA/PA.20,21 If this ratio is 1.2 or greater, it is likely the patient is a rapid acetylator. If the ratio is 0.8 or less, it is likely the patient is a slow acetylator. The Caucasian and African-American populations appear to be about evenly split between slow and rapid acetylators. Among the Japanese and Eskimo populations, 80%-90% are rapid acetylators, while only 20% or less of Egyptians and certain Jewish populations are of that phenotype. Obviously, ethnic background can play an important role in the procainamide dose required to achieve a therapeutic effect as well as the potential development of systemic lupus-like adverse effects. Metabolism of procainamide to other metabolites may be mediated by CYP2D6.22 The ratio of procainamide renal clearance and creatinine clearance equals between 2 to 3 implying that net renal tubular secretion is taking place in the kidney.18,19 The renal secretion probably takes place in the proximal tubule. Although there have been some reports that procainamide follows nonlinear pharmacokinetics, for the purposes of clinical drug dosing in patients, linear pharmacokinetic concepts and equations can be effectively used to compute doses and estimate serum concentrations.23,24
The average oral bioavailability of procainamide for both immediate-release and sustained-release dosage forms is 83%.7–11 A lag time of 20-30 minutes occurs in some patients between oral dosage administration and the time procainamide first appears in the serum. Plasma protein binding of procainamide in normal individuals is only about 15%. The recommended dose of procainamide is based on the concurrent disease states and conditions present in the patient that can influence procainamide pharmacokinetics. Procainamide pharmacokinetic parameters used to compute doses are given in the following section for specific patient profiles.
EFFECTS OF DISEASE STATES AND CONDITIONS ON PROCAINAMIDE PHARMACOKINETICS AND DOSING
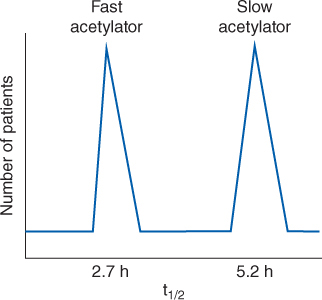
Normal adults without the disease states and conditions given later in this section and with normal liver and renal function have an average procainamide half-life of 3.3 hours (range: 2.5-4.6 hours) and a volume of distribution for the entire body of 2.7 L/kg (V = 2-3.8 L/kg; Table 8-1).25–27 N-acetyltransferase II is the enzyme responsible for conversion of procainamide to NAPA. The genetic polymorphism of N-acetyltransferase II produces a bimodal frequency distribution for procainamide half-life and clearance that separates the population into rapid and slow acetylators (Figure 8-4). The mean procainamide half-life for rapid acetylators is 2.7 hours while for slow acetylators it is 5.2 hours. Not all studies conducted with procainamide have separated results from rapid and slow acetylators when analyzing the pharmacokinetic data. Unfortunately, it is not practical to phenotype a patient as a slow or rapid metabolizer before administration of the drug, so an average population half-life and clearance is used for the purpose of initial dosage computation. Disease states and conditions that change procainamide pharmacokinetics and dosage requirements may alter clearance and the volume of distribution. The elimination rate constant (k = 0.693/t1/2, where t1/2 is the half-life) and clearance (Cl = kV) can be computed from the aforementioned pharmacokinetic parameters.
FIGURE 8-4 N-acetyltransferase II converts procainamide to its active metabolite, N-acetylprocainamide or NAPA. Patients can be phenotyped into two groups with regards to their ability to metabolize procainamide to NAPA via acetylation of the parent drug: fast acetylators convert procainamide to NAPA rapidly and have a shorter procainamide half-life, while slow acetylators convert procainamide to NAPA more slowly and have a longer procainamide half-life. This leads to a bimodal distribution of procainamide half-life for adults with normal renal function.
Because about 50% of a procainamide dose is eliminated unchanged by the kidney, renal dysfunction is the most important disease state that affects procainamide pharmacokinetics.28–30 The procainamide clearance rate decreases as creatinine clearance decreases, but this relationship is not as helpful as it is with other drugs that are primarily renally eliminated. Digoxin, vancomycin, and the aminoglycoside antibiotics are eliminated mostly by glomerular filtration. Creatinine clearance is used as an estimate of glomerular filtration rate in patients because it is relatively easy to calculate or estimate. Since the major route of renal clearance for procainamide is via proximal tubular secretion, creatinine clearance is not as reliable a parameter to aid in the estimation of procainamide clearance. In patients with renal failure, the average procainamide half-life is 13.9 hours and volume of distribution is 1.7 L/kg.
Uncompensated heart failure reduces procainamide clearance because of decreased hepatic blood flow secondary to compromised cardiac output (Table 8-2).27,28 Volume of distribution (V = 1.6 L/kg) is decreased in uncompensated heart failure patients as well. Because both clearance and volume of distribution simultaneously decrease, the increase in half-life is not as dramatic as might be expected, and patients with uncompensated heart failure have an average procainamide half-life equal to 5.5 hours (t1/2 = [0.693• ↓V]/↓Cl). The effect that uncompensated heart failure has on procainamide pharmacokinetics is highly variable and difficult to accurately predict. It is possible for a patient with uncompensated heart failure to have relatively normal or grossly abnormal procainamide clearance and half-life. For uncompensated heart failure patients, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Most clinicians reduce initial procainamide doses by 25%-50% for patients with uncompensated heart failure (Table 8-3). Patients with compensated heart failure receiving appropriate treatment with good clinical response may have normal procainamide pharmacokinetics.31 Procainamide serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with heart failure.
TABLE 8-3 Literature-Based Recommended Oral Procainamide Initial Dosage Ranges for Various Disease States and Conditions
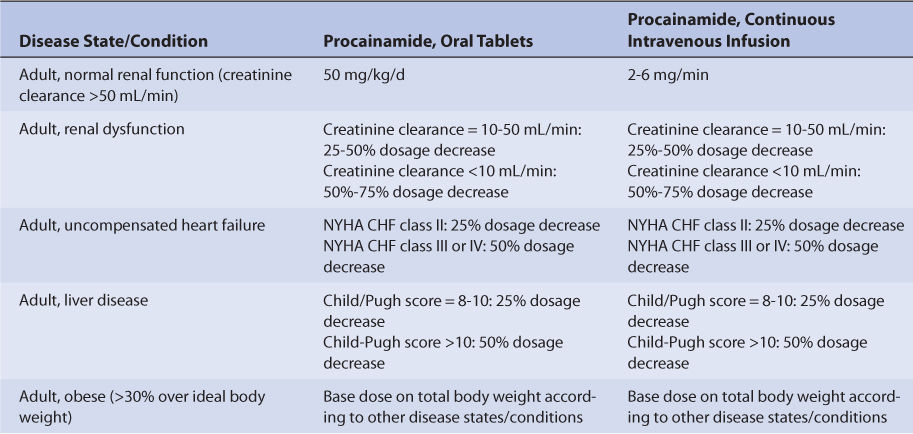
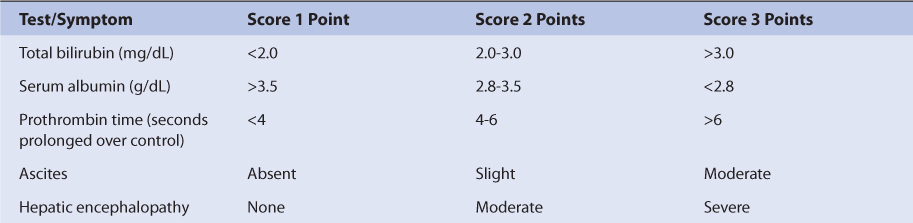
Patients with liver cirrhosis or hepatitis have not been adequately studied with regard to procainamide pharmacokinetics. However, the majority of N-acetyltransferase II responsible for the conversion of procainamide to NAPA is thought to reside in the liver. Because of this, most clinicians recommend a decrease in initial doses for procainamide in patients with liver disease.32 An index of liver dysfunction can be gained by applying the Child-Pugh clinical classification system to the patient (Table 8-4).33 Child-Pugh scores are completely discussed in Chapter 3 (Drug Dosing in Special Populations: Renal and Hepatic Disease, Dialysis, Heart Failure, Obesity, and Drug Interactions), but will be briefly discussed here. The Child-Pugh score consists of five laboratory tests or clinical symptoms: serum albumin, total bilirubin, prothrombin time, ascites, and hepatic encephalopathy. Each of these areas is given a score of 1 (normal) to 3 (severely abnormal; Table 8-4), and the scores for the five areas are summed. The Child-Pugh score for a patient with normal liver function is 5 while the score for a patient with grossly abnormal serum albumin, total bilirubin, and prothrombin time values in addition to severe ascites and hepatic encephalopathy is 15. A Child-Pugh score of 8 or more is grounds for a decrease of 25% in the initial daily drug dose for procainamide while a score greater than 10 suggests a decrease of 50% (Table 8-3). As in any patient with or without liver dysfunction, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Procainamide serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with liver cirrhosis or hepatitis.
Studies investigating the impact of obesity (30% over ideal body weight) on procainamide pharmacokinetics have found that volume of distribution correlates best with ideal body weight, but clearance correlates best with total body weight.34 The volume of distribution for procainamide should be based on ideal body weight for obese individuals according to the other disease states and conditions present in the patient. Clearance should be based on total body weight (TBW) in obese individuals (0.52 L/h/kg TBW for normal renal failure).
Procainamide is significantly removed by hemodialysis but not by peritoneal dialysis.35 Patients undergoing hemodialysis treatments may receive an additional dose of the usual amount taken after the procedure is finished. Because procainamide has a sieving coefficient equal to 0.86, continuous hemoperfusion removes significant amounts of the drug.36,37 Appropriate dosage increases should be determined using serum concentration measurements of both procainamide and NAPA.
NAPA is primarily eliminated unchanged in the urine via glomerular filtration and renal tubular secretion.18,19,25,30,38 When NAPA is given orally, 85% of the administered dose is recovered in the urine as unchanged drug. In patients with normal renal and liver function, NAPA has an average half-life of 6 hours.13 NAPA half-life increases to 41 hours on the average in patients with renal failure.30,38 The volume of distribution for NAPA in normal individuals is 1.4 L/kg. NAPA is significantly removed by hemodialysis but not by peritoneal dialysis.38 In most patients with renal dysfunction, the ratio of NAPA to procainamide steady-state concentration exceeds 1, even if the patient is a slow acetylator. The reason for this is NAPA elimination is much more dependent on renal function, so NAPA concentrations accumulate more than procainamide concentrations do in patients with renal dysfunction. Thus, in patients with renal failure, NAPA may be the predominant antiarrhythmic agent present in the serum.
DRUG INTERACTIONS
Procainamide has serious drug interactions with other drugs that are capable of inhibiting its renal tubular secretion.39–41 Cimetidine, trimethoprim, ofloxacin, levofloxacin, and ciprofloxacin are all drugs that compete for tubular secretion with procainamide and NAPA. When given with these other agents, procainamide renal clearance decreases by 30%-50% and NAPA renal clearance decreases by 10%-30%. Amiodarone increases the steady-state concentrations of procainamide and NAPA by 57% and 32%, respectively.
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate procainamide therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. Literature-based recommended dosing is a very commonly used method to prescribe initial doses of procainamide. Doses are based on those that commonly produce steady-state concentrations in the lower end of the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient.
Pharmacokinetic Dosing Method
The goal of initial dosing of procainamide is to compute the best dose possible for the patient given their set of disease states and conditions that influence procainamide pharmacokinetics and the arrhythmia being treated. In order to do this, pharmacokinetic parameters for the patient will be estimated using average parameters measured in other patients with similar disease state and condition profiles.
Half-Life and Elimination Rate Constant Estimate
Depending on the acetylator status of the patient, procainamide is almost equally metabolized by the liver and eliminated unchanged by the kidney in patients with normal hepatic and renal function. Unfortunately, there is no good way to estimate the elimination characteristics of liver-metabolized drugs using an endogenous marker of liver function in the same manner that serum creatinine and estimated creatinine clearance are used to estimate the elimination of agents that are renally eliminated by glomerular filtration. Additionally, creatinine clearance does not accurately reflect the renal elimination of procainamide because the mechanism of elimination is active tubular secretion. Because of this, a patient is categorized according to the disease states and conditions that are known to change procainamide half-life, and the half-life previously measured in these studies is used as an estimate of the current patient’s half-life (Table 8-1). For a patient with moderate heart failure (NYHA CHF class III), procainamide half-life would be assumed to equal 5.5 hours, while a patient with renal failure would be assigned an estimated half-life of 13.9 hours. To produce the most conservative procainamide doses in patients with multiple concurrent disease states or conditions that affect procainamide pharmacokinetics, the disease state or condition with the longest half-life should be used to compute doses. This approach will avoid accidental overdosage as much as currently possible. Once the correct half-life is identified for the patient, it can be converted into the procainamide elimination rate constant (k) using the following equation: k = 0.693/t1/2.
Volume of Distribution Estimate
As with the half-life estimate, the procainamide volume of distribution is chosen according to the disease states and conditions that are present (Table 8-1). The volume of distribution is used to help compute procainamide clearance, and is assumed to equal 1.7 L/kg for renal failure patients, 1.6 L/kg for uncompensated heart failure patients, and 2.7 L/kg for all other patients. For obese patients (>30% above ideal body weight), ideal body weight is used to compute procainamide volume of distribution. Thus, for a nonobese 80-kg patient without heart failure or liver disease, the estimated procainamide volume of distribution would be 216 L: V = 2.7 L/kg • 80 kg = 216 L. For a 150-kg obese patient with an ideal body weight of 60 kg and normal cardiac and liver function, the estimated procainamide volume of distribution is 162 L: V = 2.7 L/kg • 60 kg = 162 L.
Selection of Appropriate Pharmacokinetic Model and Equations
When given orally, procainamide follows a one-compartment pharmacokinetic model (see Figure 8-3). Because procainamide has such a short half-life, most patients receive oral procainamide therapy using sustained-release dosage forms. Procainamide sustained-release dosage forms provide good bioavailability (F = 0.83), supply a continuous release of procainamide into the gastrointestinal tract, and provide a smooth procainamide serum concentration-time curve that emulates an intravenous infusion when doses are given 2-4 times daily. In the United States, two different sustained-release dosage forms have been approved that provide every 6- or 12-hour dosing. Because of this, a very simple pharmacokinetic equation that computes the average procainamide steady-state serum concentration (Css in μg/mL = mg/L) is widely used and allows maintenance dosage calculation: Css = [F(D/τ)]/Cl or D = (Css • Cl • τ)/F, where F is the bioavailability fraction for the oral dosage form (F = 0.83 for most oral procainamide sustained-release products), D is the dose of procainamide in mg, and τ is the dosage interval in hours. Cl is procainamide clearance in L/h and is computed using estimates of procainamide elimination rate constant (k) and volume of distribution: Cl = kV. For example, for a patient with an estimated elimination rate constant equal to 0.210 h−1 and an estimated volume of distribution equal to 189 L, the estimated clearance would equal 39.7 L/h: Cl = 0.210 h−1 • 189 L = 39.7 L/h.
When intravenous therapy is required, a similar pharmacokinetic equation that computes the procainamide steady-state serum concentration (Css in μg/mL = mg/L) is widely used and allows dosage calculation for a continuous infusion: Css = k0/Cl or k0 = Css • Cl, where k0 is the dose of procainamide in mg/min, Cl is procainamide clearance in L/min and is computed using estimates of procainamide elimination rate constant (k), and volume of distribution: Cl = kV.
The equation used to calculate an intravenous loading dose (LD in mg) is based on a simple one-compartment model: LD = Css • V, where Css is the desired procainamide steady-state concentration in μg/mL which is equivalent to mg/L, and V is the procainamide volume of distribution. Intravenous procainamide loading doses should be infusions no faster than 25-50 mg/min to avoid severe hypotension. Two methods are used to administer procainamide loading doses. One method administers 100 mg every 5 minutes to a maximum of 500 mg; a 10 minute waiting period to allow drug distribution to tissues is utilized if more than 500 mg is needed to abate the arrhythmia. The other method administers the loading dose as a short-term infusion at a rate of 20 mg/min over 25-30 minutes, not to exceed a total dose of 17 mg/kg.
Steady-State Concentration Selection
The generally accepted therapeutic range for procainamide is 4-10 μg/mL. If procainamide + NAPA or “total procainamide” concentrations are used, the usual therapeutic range is 10-30 μg/mL, keeping in mind that procainamide and NAPA are not equipotent antiarrhythmics. However, procainamide therapy must be individualized for each patient in order to achieve optimal responses and minimal side effects.
Literature-Based Recommended Dosing
Because of the large amount of variability in procainamide pharmacokinetics, even when concurrent disease states and conditions are identified, many clinicians believe that the use of standard procainamide doses for various situations are warranted. The original computation of these doses were based on the pharmacokinetic dosing method described in the previous section, and subsequently modified based on clinical experience. In general, the procainamide steady-state serum concentration expected from the lower end of the dosage range was 4-6 μg/mL and 6-10 μg/mL for the upper end of the dosage range. Suggested procainamide maintenance doses are given in Table 8-3. A 25%-50% reduction in initial procainamide dose is suggested for patients with moderate-severe liver disease (Child-Pugh score ≥8) or moderate-severe heart failure (NYHA class II or greater). A 25%-75% decrease is indicated with renal dysfunction. When more than one disease state or condition is present in a patient, choosing the lowest daily dose will result in the safest, most-conservative dosage recommendation.
Pediatric doses are similar to those given to adults when adjusted for differences in body weight.42 The recommended intravenous loading dose is 2-6 mg/kg over 5 minutes (maximum dose 100 mg), repeating as necessary every 5-10 minutes to a maximum dose of 15 mg/kg (no more than 500 mg should be given within a 30-minute time period). For patients with ventricular tachycardia and poor perfusion, 15 mg/kg infused over 30-60 minutes as a single dose can be considered if cardioversion is ineffective. Intravenous maintenance infusion rates equal 20-80 μg/kg/min (maximum dose 2 g/d). Oral maintenance doses are 15-50 mg/kg/d. The dosage interval chosen should be appropriate for dosage form administered to the patient.
To illustrate the similarities and differences between this method of dosage calculation and the pharmacokinetic dosing method, the same examples used in the previous section will be used.
USE OF PROCAINAMIDE AND N-ACETYLPROCAINAMIDE SERUM CONCENTRATIONS TO ALTER DOSES
Because of the large amount of pharmacokinetic variability among patients, it is likely that doses computed using patient population characteristics will not always produce procainamide or NAPA serum concentrations that are expected or desirable. Because of pharmacokinetic variability, the narrow therapeutic index of procainamide, and the desire to avoid procainamide adverse side effects, measurement of procainamide and NAPA serum concentrations can be a useful adjunct for patients to insure that therapeutic, nontoxic levels are present. In addition to procainamide serum concentrations, important patient parameters (electrocardiogram, clinical signs and symptoms of the arrhythmia, potential procainamide side effects, etc) should be followed to confirm that the patient is responding to treatment and not developing adverse drug reactions.
When procainamide and NAPA serum concentrations are measured in patients and a dosage change is necessary, clinicians should seek to use the simplest, most straightforward method available to determine a dose that will provide safe and effective treatment. In most cases, a simple dosage ratio can be used to change procainamide doses, assuming the drug follows linear pharmacokinetics. Thus, linear pharmacokinetics is adequate for dosage adjustments in most patients.
Sometimes, it is useful to compute procainamide pharmacokinetic constants for a patient and base dosage adjustments on these parameters. In this case, it may be possible to calculate and use pharmacokinetic parameters to alter the procainamide dose.
In some situations, it may be necessary to compute procainamide pharmacokinetic parameters as soon as possible for the patient before steady-state conditions occur and utilize these parameters to calculate the best drug dose. Computerized methods that incorporate expected population pharmacokinetic characteristics (Bayesian pharmacokinetic computer programs) can be used in difficult cases where serum concentrations are obtained at suboptimal times or the patient was not at steady-state when serum concentrations were measured. An additional benefit of this method is that a complete pharmacokinetic workup (determination of clearance, volume of distribution, and half-life) can be done with one or more measured concentrations that do not have to be at steady-state.
Linear Pharmacokinetics Method
Because procainamide follows linear, dose-proportional pharmacokinetics in most patients, steady-state procainamide and NAPA serum concentrations change in proportion to dose according to the following equation: Dnew/Css, new = Dold/Css, old or Dnew = (Css, new/Css, old)Dold, where D is the dose, Css is the steady-state concentration, old indicates the dose that produced the steady-state concentration that the patient is currently receiving, and new denotes the dose necessary to produce the desired steady-state concentration. The advantages of this method are that it is quick and simple. The disadvantages are that steady-state concentrations are required. Because nonlinear pharmacokinetics for procainamide has been observed in some patients, suggested dosage increases greater than 75% using this method should be scrutinized by the prescribing clinician, and the risk versus benefit for the patient assessed before initiating large dosage increases (>75% over current dose).