Principles of sterilization
Susannah E. Walsh and Jean-Yves Maillard
Chapter contents
Key points
• A number of dosage forms, medical products and devices need to be free of microorganisms.
• High-level disinfection is used for the ‘sterilization’ of certain medical devices.
Introduction
Previous chapters in this Part of the book have described the types and properties of microorganisms (Chapter 13) and the action of heat and chemical agents upon them (Chapter 15). This chapter will build on those fundamentals and describe the principles underlying the different methodologies available to achieve sterility. These will be described both for pharmaceutical preparations and also for medical products and devices. This chapter will also describe the criteria used to measure sterility. The practicalities associated with the processes of sterilization are described in Chapter 17.
By definition, a sterile preparation is described as the absolute absence of viable microbial contaminants. In practice, this definition is not achievable as a preparation cannot be guaranteed to be sterile. This remark is discussed further in Chapter 17.
Certain pharmaceutical preparations, medical devices and items for which usage involves contact with broken skin, mucosal surfaces or internal organs, injection to the blood stream and other sterile parts of the body, are required to be sterile. These are frequently referred to in pharmacopoeias as sterile products or sterile dosage forms. Microbiological materials, such as soiled dressings and other contaminated items, also need to be sterilized before disposal or reuse.
Sterilization is the process by which a product is rendered sterile, i.e. the destruction or removal of microorganisms. The majority of the processes recommended by pharmacopoeias (i.e. steam under pressure, dry heat, gaseous and ionizing radiation) are terminal sterilization processes for which the preparation is sterilized in its final container or packaging. For other multiple component preparations that cannot be sterilized with such methodologies, filtration sterilization can be used. Finally, high-level disinfection is used for the ‘sterilization’ of medical devices.
Need for sterility
As mentioned in the introduction, certain pharmaceutical preparations, medical products and devices are required to be sterile (further information is given in Chapter 17, Table 17.1). Briefly, these include:
• ophthalmic preparations – eye drops, lotions and ointments and some contact lens solutions
• implants
• surgical ligatures and sutures (absorbable and non-absorbable)
Failure to achieve sterility can result in serious consequences. In the best case scenario, surviving microorganisms induce spoilage of the product (i.e. chemical and physical degradation) that might be identified before the preparation is used. The product (or batch of product) is then removed from use and destroyed. In the worst case scenario, where microbial survival cannot be identified through deleterious effects to the product, infection (sometimes fatal) might result from the use of the contaminated preparation. There have been many reports in the literature of such incidents over the years. For example, in the Devonport incident (in 1971–1972) the death of five patients (from acute endotoxic shock) was traced to dextrose 5% infusion bottles sterilized with a faulty autoclave. In 1996, in Romaira (Brazil), 35 newborn infants died of sepsis attributed to locally produced intravenous solutions (Editorial 1998). In 2005, a contaminated heparin intravenous flush was responsible for infecting several patients in different states in the USA (Editorial 2005). In these cases, inappropriate quality control procedures were implicated. These incidents emphasize that not only must an appropriate sterilization regimen be used but appropriate monitoring and control must be performed. This requires an understanding of the principles of sterilizing processes and their control and validation.
Sterilization parameters
The inactivation kinetics of a pure culture of microorganisms exposed to a physical or chemical sterilization process is generally described by an exponential relationship between the number of organisms surviving and the extent of treatment (ISO 2000), although variations from this are likely (Chapter 15 gives more details). Survivor curves have been used to generate inactivation data for specific sterilization processes using specific biological indicators (see Chapter 17). These data are important for calculating a number of sterilization parameters which help to establish a sterilizing regimen adapted to a specific preparation or product.
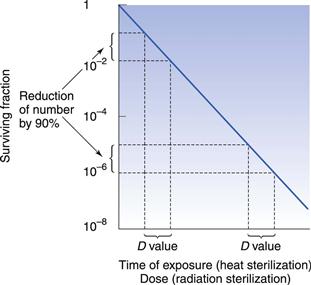
D value and Z value
One of the important concepts in sterilization is the D value (Fig. 16.1). This parameter is calculated as the time taken to achieve a one log (90%) reduction in the number of microorganisms. Another important concept is the Z value, which calculates the increase in temperature for steam (under pressure) or dry heat, or dose for radiation sterilization, required to produce a one log (90%) reduction in D value for a particular microorganism. This parameter is used to compare the heat (or dose) resistance of different biological indicators following alterations in temperature or radiation. Chapter 15 provides more information on both these parameters.
Inactivation factor and most probable effective dose
The inactivation factor is the total reduction in the number of viable microorganisms brought about by a defined sterilization process. This parameter can be calculated from the D value but only if the destruction curve follows the linear logarithmic model. To overcome problems caused by variations from this model, a most probable effective dose value can be used. This is the dose needed to achieve n decimal reductions in the number of microorganisms.
F value
The F value is a measure of total lethality of a heat sterilization process for a given microorganism and is used to compare the lethality of different heat sterilization processes. A reference value (Fo) of Geobacillus stearothermophilus (formerly Bacillus stearothermophilus) spores at 121 °C is often used with a Z value of 10 °C. The total Fo of a process includes the heating up and cooling down phases of the sterilization cycle.
For dry heat sterilization the F value concept has some limited application. The FH value is used and corresponds to the lethality of a dry heat process in terms of the equivalent number of minutes exposure at 170 °C. A Z value of 20 °C is used for the calculation.
Principles of sterilization processes
Five main types of sterilization processes are usually recommended for pharmaceutical products (British Pharmacopoeia 2012). Among these, steam sterilization (sometimes referred to as steam under pressure sterilization or high-temperature steam sterilization) still represents the gold standard. Novel sterilization processes are being developed and have already been applied in the food industry. These are mentioned in the ‘New Technologies’ section later in this chapter.
Heat sterilization
Heat has been employed as a purifying agent since early historical times, and is now used worldwide in sterilization. Boiling is not a form of sterilization as higher temperatures are needed to ensure the destruction of all microorganisms.
Microorganisms vary in their response to heat. Species of bacterial spores are thought to be some of the most heat-resistant forms of life and can survive temperatures above 100 °C. Non-sporulating bacteria are destroyed at lower temperatures (50–60 °C) and vegetative forms of yeasts and moulds have a similar response. Cysts of amoeba (e.g. Acanthamoeba polyphaga) are less sensitive than their vegetative cells which are inactivated at 55–60 °C. It is generally thought that viruses are less resistant than bacterial spores (McDonnell 2007). The agents responsible for spongiform encephalopathies, the prions, are worth mentioning due to their infectious nature and high resistance to heat (current thermal sterilization procedures are not effective in inactivating prions; see Chapter 15).
Despite the widespread use of heat sterilization, the exact mechanisms and target sites involved are still uncertain. It is likely that several mechanisms and targets are implicated and those proposed include damage to the outer membrane (Gram-negative bacteria), cytoplasmic membrane, RNA breakdown and coagulation, damage to DNA and denaturation of proteins, probably as a result of an oxidation process. For hydrated cells (steam sterilization), it is likely that the chemical lethal reactions occur more rapidly with the presence of water. The denaturation and coagulation of key enzymes and structural proteins probably result from a hydrolytic reaction.
The thermal death of bacterial cells and spores is usually thought to have a first-order reaction kinetic. Although some controversy over this does exist, the use of an exponential inactivation model for the kinetics of spores is unlikely to be underestimating the heat required (Joslyn 2001; McDonnell 2007). One way to express the order of death as a first-order reaction is:
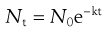 (16.1)
(16.1)
This relationship is discussed further in Chapter 15.
Heat sterilization processes usually occurs in three phases:
Principles of steam sterilization
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree