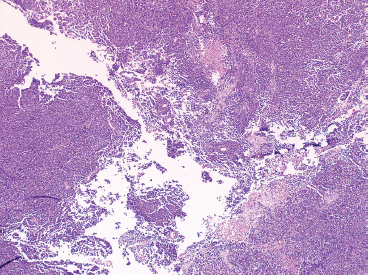
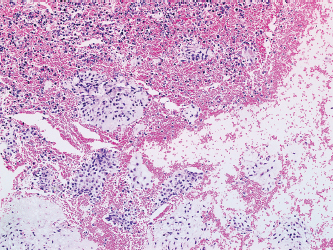
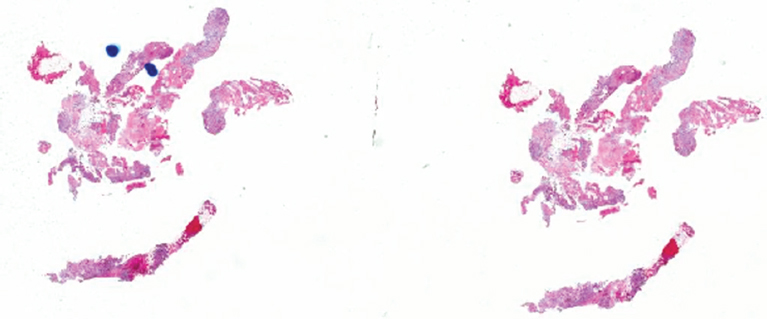
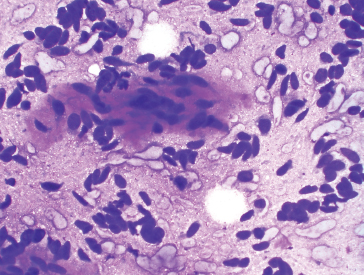
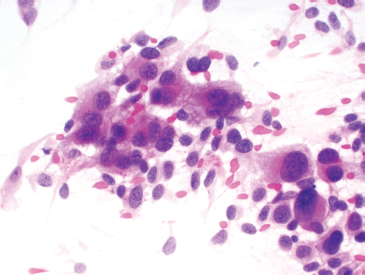
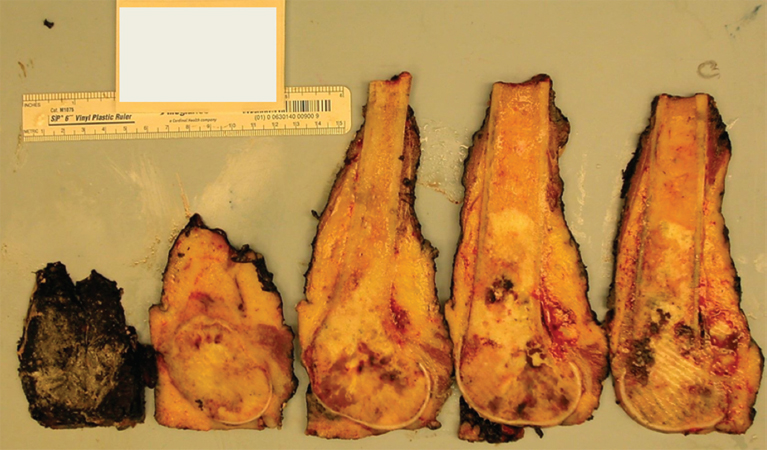
PRINCIPLES OF DIAGNOSIS 2.1 Specimen Type and Procurement 2.1 Specimen Type and Procurement For the most part, the purpose of the biopsy specimen is to determine tumor presence and type. Accurate classification of tumor type on biopsy materials is imperative and is the primary goal of obtaining a biopsy. Depending on the type of tumor, various clinical strategies can be implemented for the patient’s benefit, including observation or a “watch and wait” type of surveillance, definitive resection with either a wide or close margin, a tissue-sparing resection, or neoadjuvant therapy with a delayed resection. As such, biopsy diagnosis is one of the most critical elements for planning patient treatment. The type of biopsy technique selected will depend on a number of factors: accessibility of the lesion, size, prebiopsy likelihood of malignancy, and patient and clinician preferences. Biopsies can be either “open” or “closed.” Open biopsies involve a skin incision and surgical removal of tissue. Excisional (open) biopsies are ideal for small, superficial lesions with a low likelihood of malignancy. Incisional biopsies are less desirable overall and are often reserved for special circumstances (Figure 2.1.1). Closed biopsy systems involve small-caliber needles and include needle core biopsies and/or fine needle aspiration (Figure 2.1.2). This type of biopsy has numerous advantages over open biopsy including decreased patient discomfort and significant cost savings. Core biopsies and needle aspiration specimens are often obtained simultaneously, particularly when performed with imaging guidance. The only disadvantage of “small biopsy” specimens is that they are associated with a higher rate of inadequacy (nondiagnostic specimen), as opposed to traditional open forms of biopsy. The most essential requirement for successful biopsy diagnosis is to obtain adequate tissue for both routine microscopy as well as for potential ancillary testing. Factors that can adversely affect specimen quality include inadequate sampling, extensive tumor necrosis, intralesional hemorrhage, and for certain types of molecular testing, insufficient percentage of tumor in the specimen sample (as opposed to normal tissues). One of the best ways to ensure that high-quality material is obtained is to use rapid on-site evaluation (ROSE) for assessment of image-guided core needle biopsies. For deep-seated lesions, this represents the standard approach for an initial attempt at diagnosis in most major medical centers. The core biopsy is often preceded by a fine needle aspiration, which can be evaluated onsite while the interventionalist prepares for the next biopsy (Figures 2.1.3 and 2.1.4). Alternatively, a core biopsy can be “touched” to the slide and an immediate assessment made of the cytologic features identified on the rapid stained touch preps. As such, ROSE provides added value to the procedure in that it confirms the presence of diagnostic material and allows the cytologist onsite to “triage” specimen material into appropriate media or fixatives if ancillary studies are subsequently needed (Figure 2.1.5). From a diagnostic perspective, the information to be obtained from the examination of the resection specimen, be it excision or amputation, is very different (Figure 2.1.6). Although it is not uncommon to also be concerned about the “diagnosis” of a lesion, other factors may be equally important to consider as well. For example, the size and location of the lesion are principal features of staging and are thus essential information for the patient’s prognosis and subsequent treatment. Likewise, assessment of surgical margin status for the presence or absence of residual disease becomes an important issue, often being the prime determinant of whether or not a patient will receive adjuvant therapy. And finally, the postsurgical treatment of some bone tumors, notably osteosarcoma and Ewing sarcoma, will depend on the extent of tumor necrosis present in the resection specimen. FIGURE 2.1.1 Open, incisional biopsies often provide fragmented material. These are usually performed in limited settings: when attempts at “closed” biopsy have been unsuccessful, when an intact bone cortex precludes needle core biopsy, or when a patient cannot tolerate a needle biopsy procedure. FIGURE 2.1.2 Example of a specimen obtained through a small core cutting needle biopsy. Two levels of tissue section are provided for review. FIGURE 2.1.3 Giemsa-based stains are often used for rapid on-site assessment because they can be applied to air-dried smears very quickly. Giemsa-based stains often highlight extracellular matrix material, making them very useful for assessment of lesions of bone and soft tissue. FIGURE 2.1.4 Pap or hematoxylin and eosin stains are applied to ethanol-fixed smears and are complementary to Giemsa-based preparations. In fixed material, matrix is less obvious, but nuclear features can be seen in finer detail. FIGURE 2.1.5 Cell blocks prepared from the needle rinses of aspiration biopsy material often contain small fragments of tissue, which are appropriate for ancillary studies. FIGURE 2.1.6 Resection specimens present a different set of challenges to the diagnostician. In this setting, assessment of margin status, tumor size, and relationship to other landmarks are paramount. Ancillary testing is indispensable to the accurate classification of lesions of bone and soft tissue, particularly when dealing with small biopsy types of specimens. Many lesions can be precisely diagnosed by a combination of conventional light microscopy and judicious application of selected immunohistochemical stains. Immunohistochemistry represents a first-line diagnostic ancillary technique and can routinely be performed on formalin-fixed, paraffin-embedded tissues including core biopsies and cell block materials. In certain circumstances, immunohistochemical staining can be performed on direct smears prepared from an aspirate. This practice is not recommended as a routine procedure (immunohistochemical antibodies are usually not validated for use on direct smears), but it can be extremely helpful in circumstances where diagnostic cellular material may be limited. Prerequisites for successful performance of immunohistochemistry include well-trained personnel and a laboratory equipped with a contemporary collection of antibodies routinely used for diagnosis. These include many well-known and widely utilized markers like keratin, epithelial membrane antigen, desmin, smooth muscle actin, and S-100 protein. There are also markers that can serve as surrogates for known genetic alterations; these include CD117, friend leukemia virus integration 1 (FLI1), and isocitrate dehydrogenase 1 (IDH1). In addition, there are a number of newer markers such as transducin-like enhancer of split 1 (TLE1), discovered on GIST (DOG1), and SWI/SFF-related matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1 (SMARCB1), also known as hSNF5 and INI1, that have been derived from gene expression profiling data (Figure 2.2.1). Recognizing that there will be limitations on resources for individual laboratories, some institutions may choose to outsource some of the more esoteric markers to reference or commercial labs. These larger labs usually provide excellent service and very timely turnaround. As such, lack of direct or immediate access to some of the newer and less commonly utilized markers used in bone and soft tissue pathology should not discourage one from attempting to make a specific diagnosis. Lastly, some older, often overlooked techniques such as conventional histochemistry can have a very useful role in sarcoma diagnosis. Periodic acid-Schiff (PAS) stain, for example, can be very effective when used for specific purposes such as demonstrating the crystalline material in an alveolar soft part sarcoma or the intracellular glycogen of an Ewing sarcoma (EWS) (Figure 2.2.2). Molecular and cytogenetic information has revolutionized the field of sarcoma diagnosis. Scientific observations and research on this fascinating group of tumors has been rapidly translated into laboratory tests and techniques that can facilitate specific diagnoses. Again, these tests can be performed “in-house” if resources are available, or alternatively, “outsourced” to a reference lab. Current molecular techniques that are applicable to diagnosis of bone and soft tissue lesions can be generally divided into two categories: genome-wide screening techniques and targeted detection methods. Selection of a specific technique often requires some speculation on the suspected tumor type in order to match a “test” to a specific associated molecular or cytogenetic signature. One approach to this problem, used in many very sophisticated centers, is to simply aliquot small portions of tumor sample to a variety of media at the time of tissue procurement. This “cover all bases” approach works well with generously sized biopsies. The potential problem with this approach is that in an effort to distribute some tissue to every possible medium, one may instead “shortchange” the routine light microscopic examination. Sarcomas in general can be roughly divided into three broad categories of molecular change: (a) tumors with specific translocations, (b) tumors with specific driver mutations, and (c) tumors with complex cytogenetic abnormalities (Figure 2.2.3). Tumors with a specific translocation are best suited for targeted detection methods such as fluorescence in situ hybridization (FISH) or polymerase chain reaction (PCR). To date, approximately one-third of all sarcomas (but not sarcoma types) are characterized by a specific chromosomal translocation. A summary of some of the most common translocations and known structural abnormalities associated with lesions of soft tissue and bone are listed in Tables 2.2.1 and 2.2.2. Lesions with complex structural and numerical alterations are often better suited to genome-wide screening methods such as conventional metaphase spread karyotyping or comparative genomic hybridization. Conventional or classical cytogenetics is an excellent method of examination of the entire genome (Figure 2.2.4). This technique is well established and widely available. At present, conventional metaphase spread analysis is probably used more frequently than the more expensive and less widely available comparative genomic hybridization techniques. This may change, however, as the latter technology becomes more widely adopted and translated into routine diagnostic use. Barriers to successful incorporation of conventional cytogenetics into sarcoma diagnosis include some distinct disadvantages associated with this technique. First is the requirement for fresh tissue, a prerequisite that is easily overlooked, particularly when the neoplastic nature of a specimen is not suspected. Second, the technique is subject to many false negatives. A false-negative result may be due to either overgrowth of nontumor (stromal) cells in culture or failure of tumor to grow because of extensive necrosis. And last, karyotypes may fail to illustrate very subtle or cryptic but nevertheless diagnostically or clinically relevant important changes. Sarcomas with known translocations, the prototype being EWS and the EWS family of tumors, can be diagnostically confirmed using targeted strategies such as PCR or FISH techniques (Figure 2.2.5). Advantages of PCR include relative ease of operation and extreme sensitivity of the technique. Disadvantages include the frequent problem of specimen contamination and the need for high-quality DNA or RNA for analysis. As most laboratories use formalin-fixed, paraffin-embedded tissue as a standard for analysis, this may affect the quality of the PCR result. Depending on the fixative, time of fixation, and other factors, there may be partial degradation of nucleic acids, which can affect the recovery of DNA and RNA from tissue blocks. FISH is a nice adjunct to traditional histologic diagnosis as the morphology of the tumor is left intact (Figure 2.2.6). One disadvantage of the FISH technique is its susceptibility to test failure with strong decalcifying agents. This becomes a problem when attempting to diagnose lesions, bone tumors in particular, that need to be decalcified prior to processing. In addition, one must be careful in selecting the correct probe to “FISH for” as many of the target gene loci, EWSR1 in particular, have numerous translocation partners.
IMMUNOHISTOCHEMICAL AND HISTOCHEMICAL STAINING
MOLECULAR AND CYTOGENETIC TESTS
FLOW CYTOMETRY
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Principles of Diagnosis