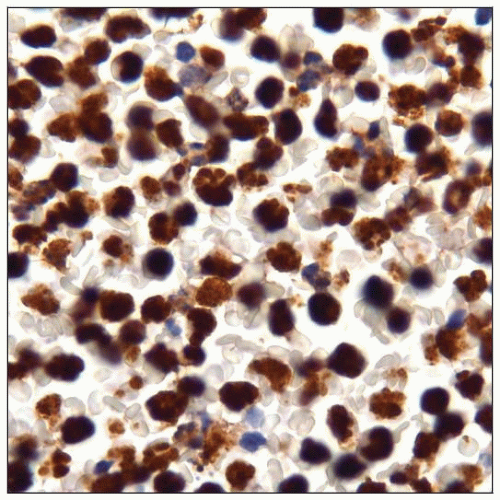
Primary Effusion Lymphoma (PEL) and Solid Variant of PEL
Francisco Vega, MD, PhD
Key Facts
Etiology/Pathogenesis
PEL arises from HHV8-infected B cells frequently coinfected by EBV
Associated with HIV infection or other severe acquired immunodeficiencies
Clinical Issues
4% of all HIV-related lymphomas
Presentation as lymphomatous growth in pleural, peritoneal, &/or pericardial effusions
Extracavitary presentation has been described
Solid variant of PEL
Some patients have coexistent Kaposi sarcoma
Microscopic Pathology
Diagnosis is usually made on cytological preparations
Cytologic features range from immunoblastic to anaplastic; plasmablastic differentiation common
Ancillary Tests
Plasma cell-associated markers(+)
Pan-B-cell markers(−)
HHV8(+) is essential for diagnosis
Top Differential Diagnoses
Diffuse large B-cell lymphoma associated with chronic inflammation
Body cavity involvement by diffuse large B-cell lymphoma, NOS
Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease
Plasmablastic lymphoma
Plasmablastic plasma cell myeloma
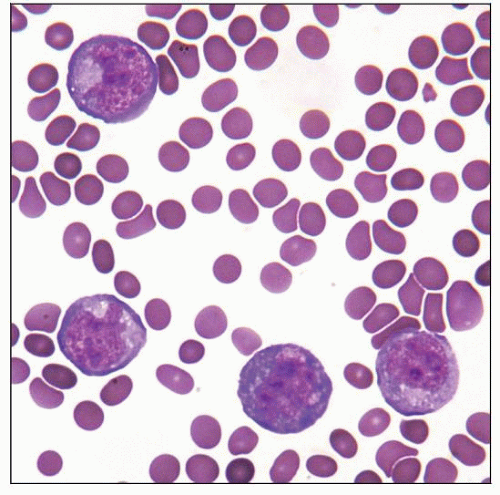 Cytospin of primary effusion lymphoma (PEL) showing medium- to large-sized lymphoid cells with abundant finely vacuolated cytoplasm and irregular nuclei. (Courtesy W. Chen, MD.) |
TERMINOLOGY
Abbreviations
Primary effusion lymphoma (PEL)
Synonyms
Body cavity-based lymphoma
Kaposi sarcoma-associated herpesvirus (KSHV)-associated lymphoma
Definitions
Human herpes virus 8 (HHV8)-associated large B-cell neoplasm most often involving body cavities
Pleural, pericardial, or peritoneal cavity
HHV8(+) lymphomas indistinguishable from PEL rarely present as solid tumor mass
These tumors are designated as extracavitary or solid variants of PEL
ETIOLOGY/PATHOGENESIS
Infectious Agents
PEL arises from HHV8-infected B cells that are frequently coinfected by Epstein-Barr virus (EBV)
HHV8 virus
γ herpes double-stranded DNA lymphotropic virus
Endemic in sub-Saharan Africa and Mediterranean region
In addition to PEL, HHV8 is associated with
Kaposi sarcoma
Multicentric Castleman disease (MCD)
MCD-associated plasmablastic lymphoma
Encodes number of homologues of cellular genes
Involved in cell proliferation and apoptosis
Clinical Associations
HIV infection or other severe acquired immunodeficiencies
Preexisting acquired immunodeficiency syndrome (AIDS) is very common
PEL also can occur in patients without immunodeficiency
Elderly patients in 8th to 9th decades in HHV8 endemic areas
Usually these tumors are EBV(−)
Rare cases of PEL are associated with hepatitis C &/or B
Pathogenesis
In PEL, B-cell differentiation program is blocked
In part due to overexpression of activated B-cell factor 1 (ABF-1) and inhibitor of differentiation 2 (ID2)
These molecules inhibit E2A (B-cell transcription factor)
E2A inhibition downregulates B-cell specific genes
Restoration of E2A activity in PEL induces apoptosis of tumor cells
CLINICAL ISSUES
Epidemiology
Incidence
Rare
0.3% of all aggressive lymphomas in HIV(−) patients
4% of all HIV-related lymphomas
Presentation
Lymphoma cells grow in pleural, peritoneal, &/or pericardial effusions
Usually no distinct extracavitary tumor masses &/or organomegaly
Frequent B symptoms
Symptoms commonly result from massive malignant effusion
Dyspnea is frequent (from pleural or pericardial disease)
Abdominal distension (from peritoneal disease)
Systemic dissemination can occur during course of disease
Associated with clinical and laboratory findings of severe immunosuppression
Marked depletion of CD4(+) T cells
Involvement of central nervous system and bone marrow is rare
Standard Ann Arbor staging is not useful as, by definition, all PEL cases are stage IV
Some patients have coexistent Kaposi sarcoma
HHV8(+) lymphomas can present as masses involving organs (extracavitary or solid variant of PEL)
Gastrointestinal tract most frequently involved
Lymph nodes can be involved
Patients with extracavitary mass often develop malignant effusion over disease course
Treatment
Highly active antiretroviral therapy (HAART) improves prognosis
Intracavitary cidofovir (antiviral agent that inhibits replication of HHV8) with interferon-α
Traditional chemotherapy, usually cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)
Bortezomib, a proteosome inhibitor that inhibits NFkB pathway
Antivirals (valganciclovir)
Rituximab probably has no role in patients with PEL
CD20 is usually negative
Prognosis
Usually poor; median survival < 6 months
IMAGE FINDINGS
Radiographic Findings
Bilateral or unilateral pleural effusion
Pericardial effusion, peritoneal effusion
Slight thickening of parietal pleura, pericardium, or peritoneum
Absence of solid tumor masses, parenchymal abnormalities, or mediastinal enlargement
MICROSCOPIC PATHOLOGY
Histologic Features
Diagnosis is usually made on cytological preparations of involved effusion fluid
Biopsy specimens of cavity lining tissue also may show small number of neoplastic cells adherent to mesothelial surfaces
Large lymphoid cells with round to irregular nuclei, prominent nucleoli, and variable morphology
Immunoblastic
Round nuclei with centrally located nucleoli
Plasmablastic
Eccentric nuclei with abundant cytoplasm, sometimes with perinuclear hof
Anaplastic
Multinucleated and Reed-Sternberg-like cells
Cytologic Features
Medium- to large-sized atypical cells, many with irregular nuclear contours, prominent nucleoli, and abundant cytoplasm (± vacuolated)
Cytomorphologic appearances ranging from immunoblastic to anaplastic and exhibiting frequent plasmablastic differentiation
ANCILLARY TESTS
Immunohistochemistry
HHV8(+) is essential for diagnosis
Plasma cell-associated markers(+)
CD138, VS38c, IRF-4/MUM1
CD38, EMA
Cytoplasmic Ig λ light chain(+/−)
CD30(+), CD45/LCA(+/−)
Notch(+) in most cases
Nuclear and cytoplasmic pattern of expression
Pan-B-cell markers(−)
CD19, CD20, CD79a, pax-5
CD15(−), LMP-1(−)
CD10(−), Bcl-6(−)
Flow Cytometry
Similar immunophenotype to that observed by immunohistochemistry
Results
CD45/LCA(+), CD71(+)
HLA-DR(+); CD23(+) in ˜ 20%
Surface Ig light chain expression is rare
CD19(−), CD20(−), CD22(−)
˜ 10% of cases have dim CD20 expression
CD10(−), FMC7(−)
Aberrant T-cell markers are positive in subset of cases
CD45RO (˜ 90%), CD7 (˜ 30%), CD4 (˜ 20%)
CD2(−), CD3(−), CD5(−), CD8(−)
Cytogenetics
Usually complex karyotype
No recurrent chromosomal abnormalities identified
In Situ Hybridization
EBER(+) in ˜ 80% of cases
Array CGH
Gains of Iq21-41, 4q28-35, 7q, 8q, 11, 12, 17q, 19p, 20q
Losses of 4q, 11q25, 14q32
Amplification of selectin-P ligand (12q24.11)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree