Primary Diffuse Large B-cell Lymphoma of the CNS
Tariq Muzzafar, MBBS
Key Facts
Clinical Issues
Neuropsychiatric signs and symptoms
Raised intracranial pressure
Intraocular involvement: blurred vision and floaters
Image Findings
Single or multiple bilateral, symmetric, periventricular lesions
Homogeneous contrast enhancement
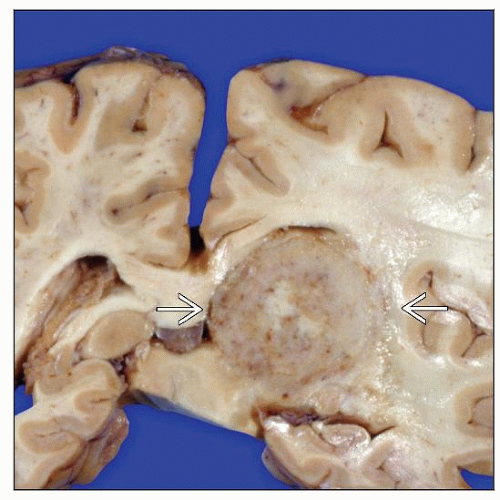
Macroscopic Features
Circumscribed masses; may be ill-defined infiltrates
Gray; granular appearing; soft consistency
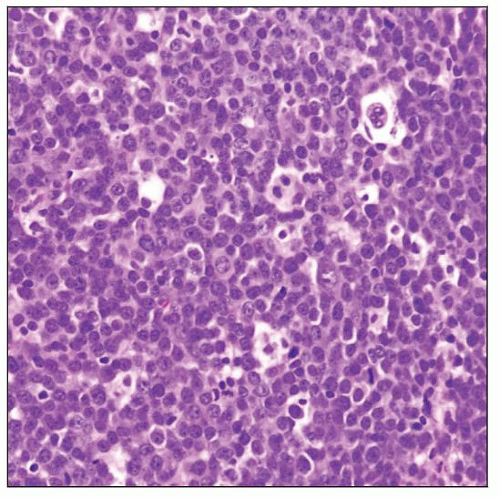
Microscopic Pathology
Diffuse pattern; may be patchy
Angiocentric and angioinvasive pattern
High-grade centroblastic morphology
Single-cell apoptosis and geographic necrosis
Intermixed cells: Small reactive lymphocytes, reactive astrocytes, foamy histiocytes
Corticosteroid effects
Extensive necrosis, sheets of macrophages
Ancillary Tests
Pan-B-cell antigens(+)
Bcl-6(+), IRF-4/MUM1(+), Bcl-2(+)
Proliferation index (Ki-67) > 50%
Monoclonal Ig gene rearrangements
Top Differential Diagnoses
Primary DLBCL of CNS associated with HIV infection
Primary intravascular lymphoma
High-grade astrocytoma
Poorly differentiated carcinoma
TERMINOLOGY
Abbreviations
Primary diffuse large B-cell lymphoma of CNS (DLBCL-CNS)
Synonyms
Primary central nervous system lymphoma
Definitions
Diffuse large B-cell lymphoma confined to central nervous system &/or intraocular location
Immunocompromised patients are excluded from this category of disease
Distinct entity in World Health Organization (WHO) 2008 classification
ETIOLOGY/PATHOGENESIS
Infectious Agents
In immunocompetent patients, there is no etiologic relationship with known viruses
Origin of Lymphoma-initiating Cells
Unknown; possibilities include
Benign systemic B cells entering CNS under physiologic conditions
Dissemination of systemic lymphoma
Extra-CNS disease eliminated by immune response, but lymphoma cells survive in immuneprivileged CNS
Molecular Heterogeneity
Features encompass spectrum of systemic DLBCL subtypes: Germinal center (GC) and activated B cell (ABC)
Germinal center origin supported by following features
Immunophenotype: CD10 &/or Bcl-6(+)
Very high load of somatic mutations of Ig genes
Mutations ongoing
May be caused by reactive T cells and antigen-presenting cells in presence of unknown antigen
ABC origin supported by following features
IgM expression
Lack of class switch recombination
Activation of NF-κB pathway
Suggested pathogenesis
Lymphoma originates from germinal center B cells destined to become IgM-expressing memory B cells
Subsequent maturation steps blocked
Possible Transforming Events
Chromosomal translocations
BCL-6 gene at chromosome locus 3q27
Correlated with shorter overall survival
Recurrent Ig gene translocations
Present in ˜ 15% of cases
Ongoing aberrant somatic hypermutation (SHM)
Increased 2-5x compared with DLBCL
6q deletions
Correlated with shorter overall survival
PRDM1 gene on 6q22-23 locus may function as tumor suppressor gene in subset of cases
Belongs to protein tyrosine phosphatase superfamily
Involved in cell contact and adhesion
Loss of protein expression in 76% of cases
Gene inactivation by DNA methylation
DAPK or MGMT
CDKN2A (P14ARF and P16INK4a)
Mutations of tumor suppressor genes
MYC, PAX-5, PIM1, Rho/TTF
Due to aberrant and ongoing somatic hypermutation
Other Factors
Role of CNS microenvironment
Not known whether B cells enter CNS as benign reactive cells or as malignant lymphoma cells
Extracerebral relapse rare
Lymphoma cell angiotropism may be due to
Interactions between homing receptors and ligands expressed by CNS endothelial cells
IGHV4-34 gene segment shows preferential usage in DLBCL-CNS
Open reading frame maintained
CNS microenvironment may favor development of lymphomas with specific Ig genes; or
Neurotropic viruses or superantigens may elicit antibodies encoded by IgHV4-34 gene segment
B cells may expand and persist in CNS
CLINICAL ISSUES
Epidemiology
Incidence
Less than 1% of all non-Hodgkin lymphomas
Approximately 2-3% of brain tumors
Incidence is reported to be increasing
Age
Median: 60 years
Gender
Slight male preponderance
Ethnicity
No ethnic predisposition
Site
In descending order: Cerebrum, cerebellum, and brainstem
Supratentorial in 60% of patients
Spinal cord in 1%
Intraocular
˜ 20% of patients with DLBCL-CNS have intraocular involvement at diagnosis
˜ 80% of patients with intraocular lymphoma develop contralateral and parenchymal CNS lesions
Ocular disease may precede clinically detectable brain lesions
Multifocal in 20-40%
Extraneural sites rarely involved
Presentation
Focal neurologic symptoms and signs in 50-80%
Psychiatric symptoms and signs in 20-30%
Seizures less frequent than in other brain tumors due to deep location
Symptoms and signs of raised intracranial pressure in ˜ 30%
Asymmetric cranial neuropathies in leptomeningeal involvement
Presents with intraocular involvement in ˜ 5%
Blurred vision and floaters
Ocular slit-lamp examination
Lymphoma cells in vitreous or retina
B symptoms (fever, night sweats, weight loss) rare
Laboratory Tests
Human immunodeficiency virus (HIV) serology is negative in DLBCL-CNS
DLBCL-CNS in HIV(+) patients is considered as separate category
Cerebrospinal fluid (CSF) analysis
Lymphoma cells identified by cytology in ˜ 25% of cases
Assessment for B-cell clonality
Flow cytometry
PCR
Serum lactate dehydrogenase (LDH) levels may be elevated
Ocular interleukin (IL)-10 levels elevated in patients with ocular involvement
Natural History
Disappearance of lesions (“ghost tumors”) can occur
Rarely spontaneous
More often corticosteroid-induced
Treatment
Options, risks, complications
Patients ≥ 60 years
Tumors demonstrate low radiosensitivity
High incidence of delayed neurotoxicity
Radiotherapy (RT) may be deferred
Refractory disease
Intensive chemotherapy (ICT) with autologous stem cell transplantation (ASCT)
Salvage treatment
ICT-ASCT may be useful
2nd-line chemotherapeutic agents
Primary intraocular lymphoma
Initial treatment similar to that for other DLBCL-CNS cases
Goal is to eradicate reservoir of disease in eye and decrease risk of recurrence
Dedicated ocular radiotherapy, intraocular chemotherapy
Surgical approaches
CNS
Biopsy for pathologic diagnosis
Resection performed only for herniation due to mass effect
Median survival following surgery alone: 1-4 months
Ocular
Biopsy of vitreous, choroid, or retina for diagnosis
Drugs
High-dose methotrexate (MTX)-based chemotherapy only as initial treatment
Highly chemosensitive tumor
Used infrequently
Combined with blood-brain barrier disruption
Delayed neurotoxicity less common
Radiation
Whole-brain radiotherapy (WBRT) alone
DLBCL-CNS is usually radiosensitive
Microscopic diffuse lesions present even in radiologically localized disease
Delayed neurotoxicity frequent
Limited survival benefit
High-dose MTX-based chemotherapy + WBRT
Median survival time: 2-4 years
5-year survival rate: 20-40%
Anti-CD20 antibodies (rituximab)
Direct intraventricular/intrathecal administration
May be useful for leptomeningeal and ocular disease
Intravenous rituximab used in combination with high-dose MTX-based chemotherapy
Prognosis
Poor prognosis of DLBCL-CNS compared with patients with systemic DLBCL may be due to
Immune-privileged location
Intrinsic aggressive biologic behavior
Several prognostic scoring systems proposed
International Extranodal Lymphoma Study Group prognostic index (0-5 scale)
Age, performance status, lactate dehydrogenase level, CSF protein, and involvement of deep structures
Nottingham/Barcelona score (0-3 scale)
Age, performance status, and extent of brain disease
Memorial Sloan Kettering Cancer Center prognostic score
Age and Karnofsky performance status score
Ocular involvement is not independent risk factor
Response to corticosteroids is favorable prognostic marker
Bcl-6 expression reported to be associated with better prognosis
IMAGE FINDINGS
General Features
Location
Single lesion in ˜ 50%
Periventricular lesions common
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree