Preservatives (type)
Example
Mechanism/property
Concern/Advantage
Detergents
Benzalkonium chloride (0.001–0.02%)
Disrupts microbial cell membrane due to its charge-related binding. Excellent disinfectant activity and wide spectrum
Disrupts corneal and conjunctival cell and induces apoptosis
Polyquaternium-1 (0.001 %)
Similar to BKC
Less toxic than BKC
Cetrimonium salt (0.005)
Similar to BKC
Corneal and conjunctival toxicity
Organomercuric compounds
Thimerosal (0.001–0.02 %)
Effective against bacteria, fungus and protozoa
Conjunctival and corneal epithelial cell toxicity reported
Phenylmercuric nitrate (0.002–0.004 %)
Similar to thimerosal
Chemical incompatibility with salts of halides
Oxidizing agents
Oxychloro complex also called as purite (mixture of chlorine dioxide, chlorite and chlorate)
Inhibit microbial protein synthesis and by oxidizing glutathione
Lesser toxic than BKC
Sodium perborate
Oxidizing microbial cell membranes and enzymes due to hydrogen peroxide generation
Lesser toxic than BKC
Ionic-buffered preservatives
SofZia
Combining antimicrobial strength of zinc, boron
Comparatively be safe for cornea but limited prevention for S. aureus
Substituted alcohols and phenols
Chlorobutanol (0.5 %)
Wider spectrum of activity on bacteria and fungus
Thermal instability, irritation on ocular surface and can cause corneal epithelial toxicity
Phenylethyl alcohol
Benzoic acid esters
Methylparaben, propylparaben
Poor antimicrobial activity
Poor water solubility, lesser toxicity as compared to BKC
Miscellaneous
Sorbates (0.1 %)
Broad-spectrum antimicrobial in lower pH
Stinging sensation and corneal irritation
EDTA (0.01–0.1 %)
Chelates divalent cations in the microbe and potentiates the effect of other preservatives
Not used alone and also chelates precorneal cations less toxic as compared to BKC
Hydroxymethylglycinate, silver chloride complex
Further studies required
Further studies required
16.3 Detergents
16.3.1 Benzalkonium Chloride
Gerhard Domagk, a German chemist who bagged a Nobel Prize for his discovery of sulphonamides (the first antimicrobials), observed that increasing nitrogen containing carbon chain length from 8 to 18 showed excellent antimicrobial activity. In 1935 he synthesized and studied the effect of benzalkonium chloride on microbes (DoMagk 1935). It was reported to have good inhibitory action of wide range of bacteria and yeast (Sevag and Ross 1944; Baker et al. 1941) After the Second World War, it was considered as nontoxic and extensively used as a cleansing agent for surgeons in the name of Zephiran and Germinal. Benzalkonium chloride (BKC) is a quaternary ammonium compound (cationic detergent) most widely used for the preservation in topical ophthalmic products. Its high water solubility and colourless and orderless nature having high thermal and chemical stability in aqueous formulations favoured its use for topical disinfectant and preservation. It is reported to be incompatible with anionic compounds such as salicylates and nitrates. It is a mixture of alkyl benzyldimethyammonium chlorides having different alkyl lengths. The highest antimicrobial activity was found to be between the alkyl group R having C16H33 to C12–H25 (Fig. 16.1). BKC has been used in the concentrations varying from 0.001 to 0.02 %. Quaternary ammonium compounds are detergents as they have a cationic polar group and non-polar carbon chain. They are reported to cause tear film instability, loss of goblet cells, conjunctival squamous metaplasia and apoptosis, disruption of the corneal epithelium barrier, and damage to deeper ocular tissues (Baudouin et al. 2010). The tear film instability could be explained by their ability to disrupt the arrangement of monolipid layer. BKC increases corneal permeability of drugs by disrupting tight junctions of corneal epithelium (Chen et al. 2012). Ocular kinetics of radiolabelled BKC has been reported to achieve higher levels in anterior ocular tissues and retained over the period of 120 h. Moreover, it has also been reported to disappear rapidly from precorneal area (Green and Chapman 1986). Most of the studies on the intraocular penetration and topical disposition studies on benzalkonium were conducted when more sensitive analytical methods like mass spectroscopy were not available; therefore, comprehensive information is not available. However, transcorneal uptake of cationic compounds through organic cation transporters (OCT) has been reported by Nirmal et al. (2013) and partially explains the fate of quaternary ammonium compounds in the precorneal area.
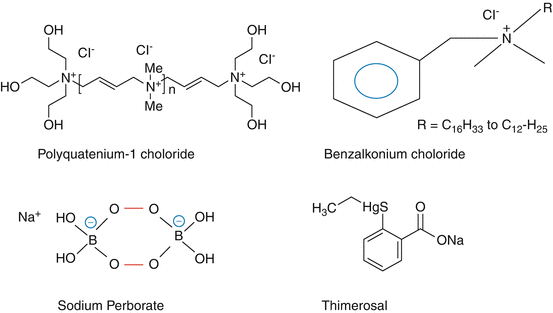

Fig. 16.1
Showing the chemical structures of benzalkonium chloride, polyquaternium (Polyquad), sodium perborate, thimerosal
Preservatives such as BKC, benzododecinium bromide, cetrimide, phenylmercuric nitrate, thimerosal (thi), methyl parahydroxybenzoate, chlorobutanol, and EDTA were compared for their cytotoxicity in human conjunctival cell line by exposing them for 15 min followed by 24 h recovery by Debbasch et al. (2001) and reported that quaternary amines are the most cytotoxic preservatives. They were found to induce apoptosis at lower concentration followed by necrosis at higher concentrations. Moreover, they have also implicated that superoxide anions may be responsible for the tissue damage caused on ocular surface.
16.3.2 Polyquaternium-1
It is a quaternary ammonium compound derivative brought by Alcon Labs as an effective preservative in multipurpose contact lens solutions. for chronic administration of travoprost in glaucoma. Studies that compared the preservatives such as Polyquad and BKC used in prostaglandin analogues on corneal surface changes reported that Polyquad showed higher degree of safety over BKC (Lee et al. 2014; Sezgin Akçay et al. 2014). Patient compliance to adhere to chronic drug regimen is high where ocular drugs related side effects are low. Although BAC is having its place as a preservative in ophthalmic products, Polyquad can be considered for chronic drug administration to have less problems with ocular surface disorder (Rolando et al. 2011; Labbé et al. 2006). Considering this safety, Polyquad has been used extensively in contact lens solutions. In this group of quaternary ammonium compounds, other members such as benzethonium chloride and cetylpyridinium chloride are also found occasionally in the drug formulations.
16.4 Organomercuric Compounds
Organomercuric compound do not share the toxicity of free mercury. Thimerosal, also called as merthiolate, is reported to have bacteriostatic, antifungal and antiprotozoal activity. It is used as a preservative in the concentration varying from 0.005 to 0.02 %. It is relatively slow acting but a stable compound used in contact lens solutions (later replaced with Polyquad and biguanides) As compared to all other preservatives, thimerosal 0.004 % when combined in solution with EDTA was effective against Acanthamoeba castellanii and Acanthamoeba polyphaga trophozoites and cysts (Silvany et al. 1991). Phenylmercuric nitrate or acetate is used in the concentration varying from 0.002 to 0.004 % in ophthalmic solutions. It is reported to have considerable antifungal activity, and its in vitro effect is significantly superior to those of benzalkonium chloride, natamycin and ketoconazole against ocular pathogenic filamentous fungi (Xu et al. 2013). Phenylmercuric nitrate has been reported to have incompatibility with halide salts of other compounds in the formulation.
16.5 Benzoic Acid Esters
Methyl- and propylparabens are rarely used as a preservative in ophthalmic solutions. Methylparaben is used in the concentration varying from 0.1 to 0.2 %. They show poor antimicrobial activity. Ocular irritation and stinging have been reported.
16.6 Phenols and Substituted Alcohols
16.6.1 Chlorobutanol
It is reported to have wider spectrum over Gram-positive and Gram-negative bacteria, P aeruginosa and fungi. It is used at the concentration of 0.5 % in the topical solutions. It is reported to undergo decomposition while on standing and at higher temperatures causing release hydrochloric acid which in turn reduces the pH of the aqueous solution. Therefore, the solution is buffered at 5–5.5 to improve its shelf life.
16.7 Oxidative Preservatives
16.7.1 Stabilized Oxychloro Complex
Stabilized oxychloro complex (SOC) is a preservative used at a low concentration such as 0.005 % in ophthalmic solutions. It is reported to have a broad spectrum of activity. This oxychloro complex is a mixture containing chlorine dioxide, chlorate and chlorite. It is reported to inhibit microbial protein synthesis and by oxidizing glutathione. Light causes SOC dissociation into chloride, oxygen, chlorine and sodium species. Chloride free radicals are involved in the oxidation process causing microbial kill (Noss and Olivieri 1985). Oral administration of 5 mg/L (0.0005 %) solution of SOC for 12 weeks was tested in human volunteers and was found to be safe (Lubbers et al. 1982).
16.7.2 Sodium Perborate
It was introduced in 1950 as a disinfectant for oral rinse and dental bleach (Saenz 1950). It is a odourless water-soluble sodium salt having a chemical formula Na2H4B2O8 (Fig. 16.1). It is prepared by mixing sodium borate with hydrogen peroxide in alkaline condition and available as hydrate in crystalline form. In aqueous solution, it releases hydrogen peroxide and borate, but it has been found to have an equilibrium with peroxoborate anion (McKillop and Sanderson 1995). It is considered as less toxic as compared to BKC in experimental studies.
16.7.3 sofZia Proprietary Ionic Buffer
SofZia is a preservation system developed by Alcon Laboratories as a proprietary ionic buffer system (Fort Worth, Texas) for antiglaucoma medication travoprost. This preservation system having composition containing borate, sorbitol, propylene glycol and zinc (Noecker 2007) has been reported to meet the requirement of US Pharmacopoeia standards for ophthalmic preservatives. This composition containing ingredients were all found to be safe for ocular use; therefore, it has been promoted for chronic drug therapy for glaucoma and dry eye (Rosenthal et al. 2006). Many studies found that preservation with SofZia composition equals effect with low toxicity on the ocular surface (Anwar et al. 2013). Use of boric acid in eye wash has been known for a long time, and antimicrobial action on pathogenic organisms of the eye was reported in the concentrations from 0.5 to 2 % solutions by Novak and Taylor in 1951. Similarly, antimicrobial activity of zinc salts has also been well known (Choi et al. 2010). Together with all at a given pH, SofZia has been reported to be effective as per US Pharmacopeia standards for ocular drug preservation. When travoprost with sofZia and BKC was compared, it has been found that SofZia did not meet European Pharmacopoeia criteria due to its limited effectiveness against Staphylococcus aureus. However, both products satisfied US and Japanese Pharmacopoeia criteria (Ryan et al. 2011).
16.7.4 Studies Comparing of Preservatives in Terms of Efficacy and Toxicity
Many studies are available comparing the toxicity of various preservatives; however, lack of uniformity in the dose, variation in the methods adopted for testing and diverse experimental conditions are the factors making it difficult to have head-to-head comparison among them. A study assessed the effect of drug preservatives such as benzalkonium chloride, Polyquad, purite and sofZia-like mixture on trabecular meshwork cells. This study reported that BKC and Polyquad caused significant DNA damage, cell viability and increased DNA fragmentation. BKC, Polyquad and purite all caused altered gene expression. However, all of these effects were found to be less by sofZia (Izzotti et al. 2015). Long-term use of topical drugs in conditions like glaucoma is reported to induce toxic immunopathological changes in the ocular surface. One-month topical administration of preservatives cetrimonium chloride (0.01 %), benzalkonium chloride (0.01 %), benzododecinium bromide (0.01 %), thiomersal (0.004 %) and methyl parahydroxybenzoate (0.05 %) were all reported to cause similar changes in rat cornea (Becquet et al. 1998). When sodium chlorite was compared with BKC, based on the depletion of intracellular glutathione in conjunctiva and corneal epithelial cells, Ingram et al. (2004) reported that best balance of high antibacterial toxicity with low ocular toxicity was achieved with sodium chlorite. Another study compared the toxicity of preservatives on cell viability in immortalized human conjunctival and corneal epithelial cells; this study concluded that the toxicity was found from higher to low in the order of thimerosal 0.0025 % > BKC (0.025 %) > chlorobutanol (0.25 %) > methylparaben (0.01 %) > sodium perborate (0.0 025 %) and EDTA (0.01 %). For long-term administration, BKC showed unfavourable outcome in most of the studies, but substitution of BAK with Polyquad or sofZia has resulted in significant improvement in conjunctival and corneal cells (Ammar et al. 2010).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


