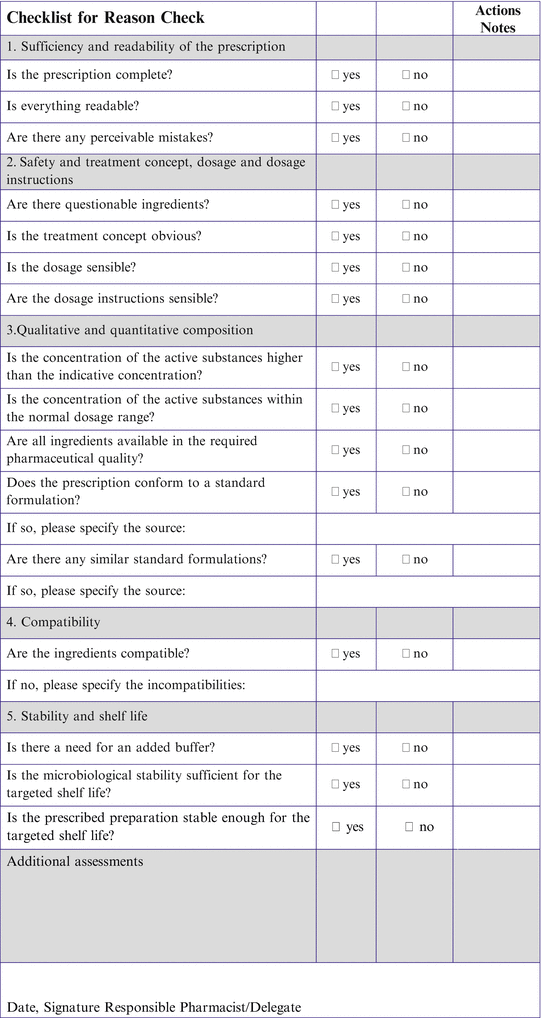
Fig. 2.1
Request form for a non-catalogue extemporaneous product
This process creates an appropriate barrier to pharmacists who might otherwise decide to authorise ad hoc or unusual formulations without considering the associated risks. The group of senior pharmacists and technicians then meet every few months to review the requests for non-catalogue preparations, and review whether other options should be pursued e.g. purchase of a licensed product from a foreign country, use of a batch-manufactured product rather than an extemporaneously-prepared product for an individual etc.
As a guide to help the risk assessment, the department provides a risk assessment matrix to highlight potential problems to the clinical pharmacist, see Fig. 2.2.
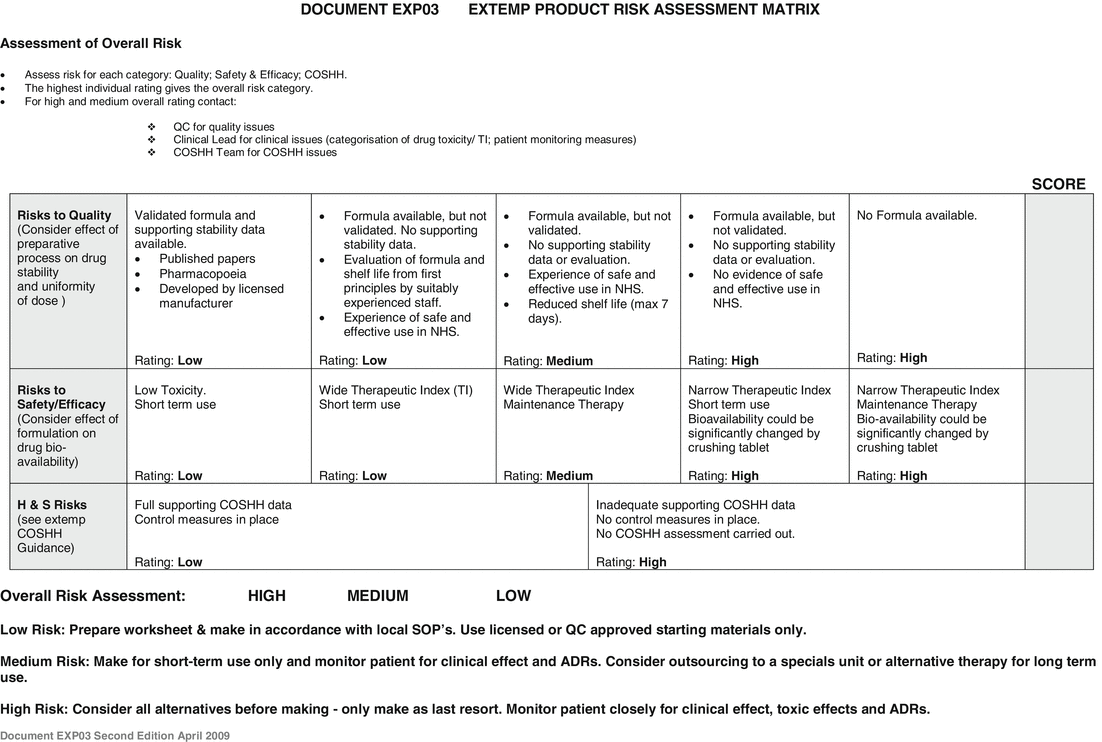

Fig. 2.2
Extemporaneous product risk assessment matrix
2.2.3.2 German Reason Check
In Germany, pharmacists must perform ‘reason checks’ (Plausibilitätsprüfung) to establish the likely safety, quality, and efficacy of the product they want to prepare. The Pharmacy Practice Order (Apothekenbetriebsordnung, ApBetrO) specifies parameters of the preparation formula that must be checked, whether it is a doctor’s prescription or self-medication on patient’s request. Beyond that, the guideline recommends that the pharmacist considers the overall rationale for treatment.
A form has been developed (Fig. 2.3) for the performance and documentation of the reason check. Some parts will be dealt with.
Regarding “Qualitative and quantitative composition”: Substances used as active substance or as an excipient in pharmaceutical preparations have to be described in an individual monograph of the European Pharmacopoeia or comply with the requirements of the relevant general monographs (see also Sect. 23.1). Cosmetics and medicinal products may only be used if the required quality is documented. In Germany it is not allowed to change or add any active substances without permission of the prescriber. This does not apply to excipients that have no pharmacological effect.
Regarding “Compatibility”: if components of a prescribed preparation are not compatible (or there is a lack of evidence), it does not mean automatically that it should not be prepared. The preparation needs to show sufficient compatibility up to the in use expiry date; it may be possible to produce a preparation with a shortened but useful shelf life. Interactions between the active substances and excipients can however make it impossible to produce a preparation of sufficient quality. These incompatibilities can be visible or invisible during preparation. The attending pharmacist has to verify if incompatibilities are apparent. More information about incompatibilities is to be found in references such as Fiedler (Sect. 39.2.2), Handbook of Pharmaceutical Excipients (Sect. 39.2.3), Martindale (Sect. 39.2.4), Handbook of Extemporaneous Preparation (Sect. 39.4.6), Kommentar zum Arzneibuch (Sect. 39.4.8) and Trissel’s Stability of Compounded Formulations (Sect. 39.4.14).
Regarding “Stability and shelf life”: These items are amply discussed in Chap. 22 Stability. Stability is influenced by the solubility of all substances in the preparation, pH of the base, pH at which the active substances are stable, and the influence of oxygen and light. Shelf life is restricted due to:
Chemical, physical, physico-chemical reactions
Rheological changes
Formations of toxic degradation products
Microbiological growth
Decrease in concentration of the active substance
Incompatibilities or issues caused by the container
2.2.3.3 Risk-Benefit Form [4]
A risk-benefit form has been elaborated for extemporaneous and for stock preparation (Figs. 2.4 and 2.5). They enable the pharmacist to list and balance the benefits and risks of the clinical and pharmaceutical qualities of the required pharmacy preparations. The form follows the process for handling of requests for preparation, and defines decisive steps, levels of evidence of decisions, individuals concerned and responsibilities.
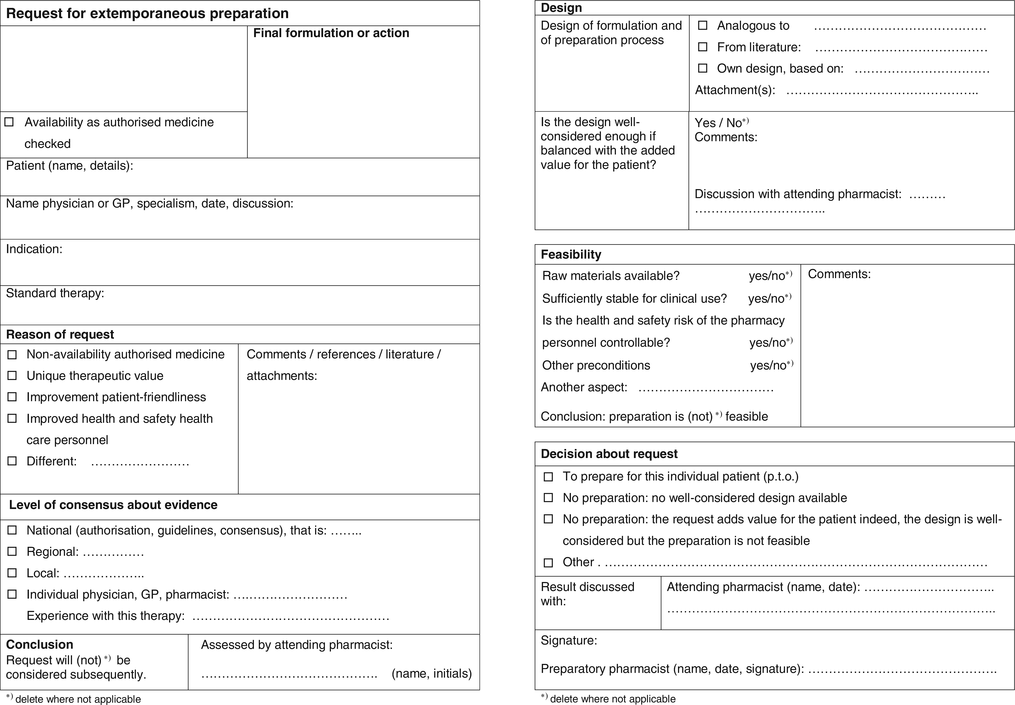
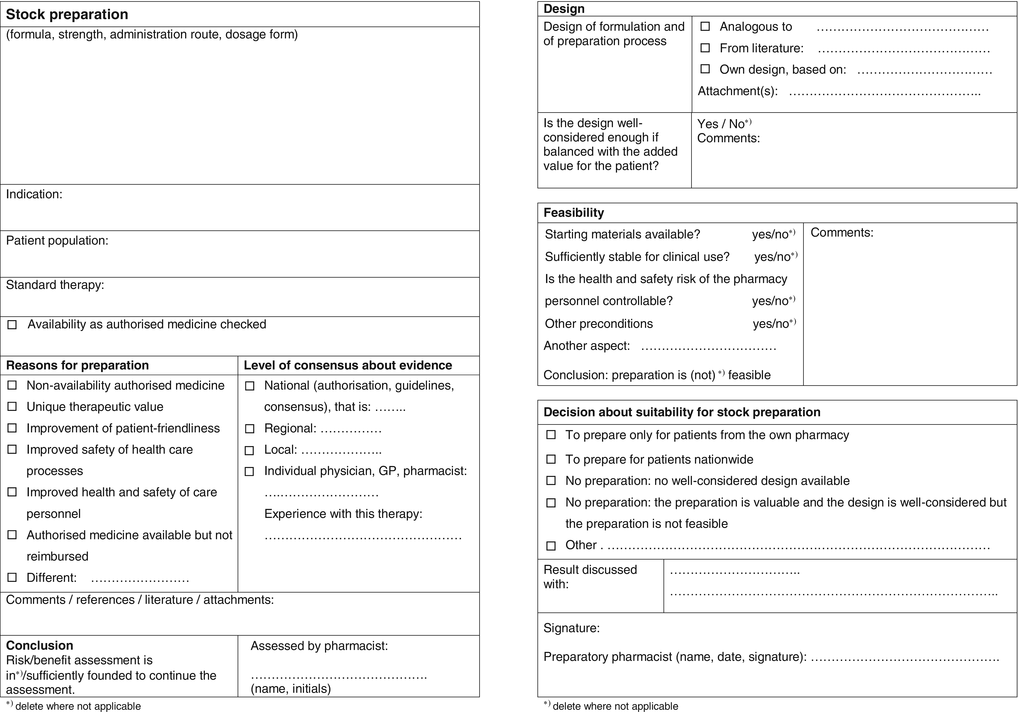

Fig. 2.4
Form for balancing risks and benefits of an extemporaneous preparation; front and back side (see Sect. 2.2.3.3)

Fig. 2.5
Form for balancing risks and benefits of a stock preparation; front and back side (see Sect. 2.2.3.3)
Possible benefits include:
Unique therapeutic value if there is no comparable authorised medicine available
Improved patient friendliness and therefore a better compliance with therapy
Improved safety of health care processes (using preparations that don’t need any reconstitution steps on the wards or in nursing homes)
Improved occupational safety and health (OSH) of health care personnel (by diminishing exposure from hazardous active substances)
(Lower price)
Possible risks include:
Uncertainty about therapeutic safety and efficacy
Design failure causing quality defects, like poor bioavailability or poor content uniformity
Preparation risk: if the actual pharmaceutical quality system cannot guarantee that the preparation will fully meet specifications
Discouraging the marketing of authorised of medicines
The forms for extemporaneous and stock preparation use the same benefits and risks. With extemporaneous preparations the balance refers to an individual patient. With stock preparations the balance results in the definition of the group of (anonymous) patients for whom, or care situation in which, the benefits may outweigh the risks.
Clinical benefits and risks are assessed on the front of the form by the attending pharmacist, who decides if the request adds enough value to be considered further. On the back the preparatory pharmacist assesses the risks of design and preparation. He also checks the feasibility: if necessary conditions are met such as availability of starting materials or sufficient control of the health and safety risk of the pharmacy personnel. Over-all it is the preparatory pharmacist who decides:
In case of an extemporaneous preparation if he accepts the request(or not)
In the case of a stock preparation on the conditions on which he will make this preparation available.
Balancing benefits and risks (see Fig. 2.6) is not a matter of mathematics but of professionalism, responsibility and transparency. The forms therefore are transparent about the decisions and show who made them.
2.3 The Prescription
2.3.1 Legal Requirements
A prescription is a request from a prescriber (usually a doctor) to a pharmacist to dispense a medicine in the stated amount, strength and method of use. Each country has its own medicines law which will define the exact requirements of a prescription. However, there is a standard data set used with the European Economic Area (European Union plus Lichtenstein, Norway and Switzerland) as given in Table 2.1.
Identification of the patient | Surname(s) |
|---|---|
First name(s) (written out in full, i.e. no initials) | |
Date of birth | |
Authentication of the prescription | Issue date |
Identification of the prescribing health professional | Surname(s) |
First name(s) (written out in full, i.e. no initials) | |
Professional qualification | |
Details for direct contact (email and telephone or fax, the latter both with international prefix) | |
Work address (including the name of the relevant Member State) | |
Signature (written or digital, depending on the medium chosen for issuing the prescription) | |
Identification of the prescribed product, where applicable | ‘Common name’ as defined by Article 1 of Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use |
The brand name if: | |
(a) the prescribed product is a biological medicinal product, as defined in point 3.2.1.1.(b) of Annex I (Part I) to Directive 2001/83; or | |
(b) the prescribing health professional deems it medically necessary; in that case the prescription shall shortly state the reasons justifying the use of the brand name | |
Pharmaceutical formulation (tablet, solution, etc.) | |
Quantity | |
Strength, as defined in Article 1 of Directive 2001/83/EC | |
Dosage regimen |
In some countries, the list of prescribers may include ‘non-medical prescribers’ such as nurses, pharmacists or chiropodists. These other prescribers may have limited formularies in some countries. However, the law varies between different countries; the validating pharmacist must takes steps to assure themselves that the prescriber is appropriately registered to prescribe.
The pharmacist should consult the prescriber if it is possible or more appropriate to use a different medicine. A licensed medicinal product should be used in preference to a pharmacy preparation, if an appropriate product is available.
When a pharmacist considers that the delivery of a medicine carries an unacceptable level of risk, he can refuse to dispense the medicine to the patient, as pharmacists have a duty of care to the patient. In this situation, they must contact the prescriber to discuss possible alternatives.
2.3.2 Consultations with the Prescriber and the Patient
2.3.2.1 Consultation About a Prescription
When there are doubts about the pharmaceutical options, consultation between the pharmacist and the prescriber takes place. The pharmacist can advise on the options for treatment following consideration of the diagnosis and pathophysiology by the doctor.
A discussion between the pharmacist and the patient (or carer) may also be needed in order to make the most appropriate treatment decision. This is often the case in paediatrics, and the parent or carer may require some assurances about the need for medication, especially if the treatment is long term.
Adherence to treatment regimens is influenced by a number of other factors, including the formulation (e.g. solid or liquid), administration route (e.g. rectal or oral), dosage size and frequency, and the organoleptic qualities of the medicine chosen (e.g. smell, appearance, flavour). By choosing a formulation that is easy to administer and by giving good information and instruction, the patient is more likely to comply with their treatment regimen.
Case – Vitamin ADEK Mixture
Pim is 14 years old and suffers from cystic fibrosis. Due to his illness, he cannot absorb fat-soluble vitamins well. The paediatrician recommends that Pim needs treatment with vitamin A, D, E and K in various oral preparations. The licensed pharmaceutical preparation consisting of 400 IU vitamin D is no longer available, meaning Pim potentially has to take even more tablets than previously.
The parents express concern to the paediatrician that Pim is unlikely to comply with his treatment. Therefore, the paediatrician requests a pharmacy preparation in which all vitamins are combined.
The pharmacist designs an oral liquid based on a standard formulation for an oral vitamin D solution. The mixture contains, per millilitre, 750 IU vitamin A, 250 IU vitamin D, 50 mg vitamin E and 0,25 mg vitamin K.
The pharmacist chooses an aqueous solution, because of the better availability of fat-soluble vitamins in patients with absorption disorders, such as cystic fibrosis patients. Due to a lack of data about the shelf life of the mixture and the absence of a stability-indicating analytical assay, a shelf life of 1 month in the refrigerator is assigned.
Pim now only has to use daily 4 mL of the oral solution that the pharmacy prepares for him every month.
Various national formularies exist and may be useful to consult during discussions with the relevant doctor e.g the Dutch national child formulary (www.kinderformularium.nl) [6] contains various pharmacy preparations, which are included in the Dutch pharmacists Formulary (FNA, see Sect. 39.4.5).
Such national formularies are good starting points for formulations as they may have been tested or supported by published or validated formulae. A second example is the German Formulary (Neues Rezeptur-Formularium, see Sect. 39.4.2). In the UK, the Handbook of Extemporaneous Preparation (see Sect. 39.4.6) also lists a selection of 50 commonly used formulae, and the British Pharmacopoeia has a small number of formulations detailed. The existence of validated formulae then allows for the potential for batch manufacture and suitable quality control testing.
Another advantage of using a national formulary is that it is kept up to date, with obsolete formulations removed or replaced regularly. This may be due to a change in recommendations (e.g. an excipient is no longer considered appropriate) or when a suitable licensed formulation becomes available.
Ferrous chloride oral drops had been removed from the Dutch formulary as there are now sufficient alternatives like Ferrous fumarate oral suspension 20 mg/ml as a licensed pharmaceutical preparation. That product however is so viscous that a small volume, which is necessary for young children, cannot be measured accurately. In the FNA therefore a Ferrous chloride oral solution 45 mg/ml has been reintroduced. This oral solution contains 20 mg iron (II) per ml which is suitable for children and it is not viscous. So the necessary small amounts can be easily measured.
2.3.3 Dose
The doctor writes the dose on the prescription and the pharmacist checks or ‘validates’ this dose. The validation process may take place with the help of a pharmacy computer system or electronic prescribing system. The usual support offered by pharmacy computer systems is limited if local formulae are used, and the pharmacist may need to consult a range of reference sources when considering the appropriate indications, doses, likely side effects and contra-indications. Extra care is required with some patient populations, such as children and the elderly.
2.3.3.1 Dosage Expression
The way in which the prescriber writes the dose is dependent on the administration form. Capsules and suppositories are given in an amount (usually milligrams) per dose unit followed by the number of units and the daily or weekly dose. For example:
R/ Folinic acid capsules 10 mg
x 10
1 capsule once a week
In the case of oral liquids, the doctor will write the strength in milligrams per millilitre followed by the amount and dose. In the case of electrolytes the strength is often written in millimol per millilitre because the dosages of electrolyte are based on blood concentrations. For example:
R/ Magnesium gluconate oral solution 0,1 mmol/mL