KEY CONCEPTS
![]() Complex physiology surrounds the process of fertilization and pregnancy progression.
Complex physiology surrounds the process of fertilization and pregnancy progression.
![]() Drug characteristics and physiologic changes modify drug pharmacokinetics during pregnancy, including changes in absorption, protein binding, distribution, and elimination, requiring individualized drug selection and dosing.
Drug characteristics and physiologic changes modify drug pharmacokinetics during pregnancy, including changes in absorption, protein binding, distribution, and elimination, requiring individualized drug selection and dosing.
![]() Although drug-induced teratogenicity is a serious concern during pregnancy, most drugs required by pregnant women can be used safely. Informed selection of drug therapy is essential.
Although drug-induced teratogenicity is a serious concern during pregnancy, most drugs required by pregnant women can be used safely. Informed selection of drug therapy is essential.
![]() Healthcare practitioners must know where to find and how to evaluate evidence related to the safety of drugs used during pregnancy and lactation.
Healthcare practitioners must know where to find and how to evaluate evidence related to the safety of drugs used during pregnancy and lactation.
![]() Health issues influenced by pregnancy, such as nausea and vomiting, can be treated safely and effectively with nonpharmacologic treatment or carefully selected drug therapy.
Health issues influenced by pregnancy, such as nausea and vomiting, can be treated safely and effectively with nonpharmacologic treatment or carefully selected drug therapy.
![]() Some acute and chronic illnesses pose additional risks during pregnancy, requiring treatment with appropriately selected and monitored drug therapies to avoid harm to the woman and the fetus.
Some acute and chronic illnesses pose additional risks during pregnancy, requiring treatment with appropriately selected and monitored drug therapies to avoid harm to the woman and the fetus.
![]() Management of the pregnant woman during the peripartum period can encompass uncomplicated pregnancies/deliveries, but can also include a wide variety of potential complications that require use of evidence-based treatments to maximize positive maternal and neonatal outcomes.
Management of the pregnant woman during the peripartum period can encompass uncomplicated pregnancies/deliveries, but can also include a wide variety of potential complications that require use of evidence-based treatments to maximize positive maternal and neonatal outcomes.
![]() Understanding the physiology of lactation and pharmacokinetic factors affecting drug distribution, metabolism, and elimination can assist the clinician in selecting safe and effective medications during lactation.
Understanding the physiology of lactation and pharmacokinetic factors affecting drug distribution, metabolism, and elimination can assist the clinician in selecting safe and effective medications during lactation.
A controversial and emotionally charged subject because of medicolegal and ethical implications, drug use in pregnancy and lactation is a topic often underemphasized in the education of health professionals. Clinicians are responsible for ensuring safe and effective therapy before conception, during pregnancy and parturition, and after delivery. Active patient participation is essential. Optimal treatments of illnesses during pregnancy sometimes differ from those used in the nonpregnant patient.
In many cases, medication dosing recommendations for acute or chronic illnesses in pregnant women are the same as for the general population. However, some cases require different dosing and selection of medications. Principles of drug use during lactation, although similar, are not the same as those applicable during pregnancy.
PHYSIOLOGY OF PREGNANCY
![]() Fertilization and progression of pregnancy are complex, resulting in survival of only approximately 50% of embryos.1 Because most losses occur early, usually in the first 2 weeks after fertilization, many women do not realize they were pregnant. Spontaneous loss of pregnancy later in gestation occurs in about 15% of pregnancies that survive the first 2 weeks after fertilization.2
Fertilization and progression of pregnancy are complex, resulting in survival of only approximately 50% of embryos.1 Because most losses occur early, usually in the first 2 weeks after fertilization, many women do not realize they were pregnant. Spontaneous loss of pregnancy later in gestation occurs in about 15% of pregnancies that survive the first 2 weeks after fertilization.2
Fertilization occurs when a sperm attaches to the outer protein layer of the egg, the zona pellucida, and renders the egg non-responsive to other sperm.3 The attached sperm releases enzymes that allow the sperm to fully penetrate the zona pellucida and contact the egg’s cell membrane. The membranes of the sperm and egg then combine to create a new, single cell called a zygote. Male and female chromosomes join in the zygote, fuse to create a single nucleus, and organize for cell division.4
Fertilization usually occurs in the fallopian tube.4 The fertilized egg travels down the fallopian tube over 2 days, with cell division taking place. By day 3, the fertilized egg reaches the uterus. Cell division continues for another 2 to 3 days in the uterine cavity before implantation. Approximately 6 days after fertilization, the cell mass is termed a blastocyst. Human chorionic gonadotropin (hCG) now is produced in amounts detectable by commercial laboratories. Implantation begins with the blastocyst sloughing the zona pellucida to rest directly on the endometrium allowing initiation of growth into the endometrial wall. By day 10 postfertilization, the blastocyst is implanted under the endometrial surface and receives nutrition from maternal blood.4 Now it is called an embryo.5
After the embryonic period (between weeks 2 and 8 postfertilization), the conceptus is renamed a fetus.6 Most body structures are formed during the embryonic period, and they continue to grow and mature during the fetal period. The fetal period continues until the pregnancy reaches term, approximately 40 weeks after the last menstrual period.5
Gravidity is the number of times that a woman is pregnant.6,7 A multiple birth is counted as a single pregnancy. Parity refers to the number of pregnancies exceeding 20 weeks’ gestation and relates information regarding the outcome of each pregnancy. In sequence, the numbers reflect (a) term deliveries, (b) premature deliveries, (c) aborted and/or ectopic pregnancies, and (d) number of living children.7 A woman who has been pregnant four times; has experienced two term deliveries, one premature delivery, and one ectopic pregnancy; and has three living children would be designated G4P2113.
Characteristics of Pregnancy
Pregnancy lasts approximately 280 days (about 40 weeks or 9 months); the time period is measured from the first day of the last menstrual period to birth.6,7 Gestational age refers to the age of the embryo or fetus beginning with the first day of the last menstrual period, which is about 2 weeks prior to fertilization. When calculating the estimated due date, add 7 days to the first day of the last menstrual period then subtract 3 months. Pregnancy is divided into three periods of 3 calendar months, each called a trimester.
Early symptoms of pregnancy include fatigue and increased frequency of urination. At approximately 6 weeks’ gestation, nausea and vomiting can occur. While commonly called morning sickness, it can happen at any time of the day. Nausea and vomiting usually resolve at 12 to 18 weeks’ gestation. A pregnant woman can feel fetal movement in the lower abdomen at 16 to 20 weeks of gestation. Signs of pregnancy include cessation of menses, change in cervical mucus consistency, bluish discoloration of the vaginal mucosa, increased skin pigmentation, and anatomic breast changes.6,7
Pharmacokinetic Changes During Pregnancy
![]() Normal physiologic changes that occur during pregnancy may alter medication effects, resulting in the need to more closely monitor and, sometimes, adjust therapy. Physiologic changes begin in the first trimester and peak during the second trimester. For medications that can be monitored by blood or serum concentration measurements, monitoring should occur throughout pregnancy.
Normal physiologic changes that occur during pregnancy may alter medication effects, resulting in the need to more closely monitor and, sometimes, adjust therapy. Physiologic changes begin in the first trimester and peak during the second trimester. For medications that can be monitored by blood or serum concentration measurements, monitoring should occur throughout pregnancy.
During pregnancy, maternal plasma volume, cardiac output, and glomerular filtration increase by 30% to 50% or higher, potentially lowering the concentration of renally cleared drugs.8,9 As body fat increases during pregnancy, the volume of distribution of fat-soluble drugs may increase. Plasma albumin concentration decreases, which increases the volume of distribution of drugs that are highly protein bound. However, unbound drugs are more rapidly cleared by the liver and kidney during pregnancy, resulting in little change in concentration. Hepatic perfusion increases, which could theoretically increase the hepatic extraction of drugs. Nausea and vomiting, as well as delayed gastric emptying, may alter the absorption of drugs. Likewise, a pregnancy-induced increase in gastric pH may affect the absorption of weak acids and bases. Higher levels of estrogen and progesterone alter liver enzyme activity and increase the elimination of some drugs but result in accumulation of others.8,9
Transplacental Drug Transfer
![]() Although once thought to be a barrier to drug transfer, the placenta is the organ of exchange for a number of substances, including drugs, between the mother and fetus. Most drugs move from the maternal circulation to the fetal circulation by diffusion.10 Certain chemical properties, such as lipid solubility, electrical charge, molecular weight, and degree of protein binding of medications, may influence the rate of transfer across the placenta.
Although once thought to be a barrier to drug transfer, the placenta is the organ of exchange for a number of substances, including drugs, between the mother and fetus. Most drugs move from the maternal circulation to the fetal circulation by diffusion.10 Certain chemical properties, such as lipid solubility, electrical charge, molecular weight, and degree of protein binding of medications, may influence the rate of transfer across the placenta.
Drugs with molecular weights less than 500 Da readily cross the placenta, whereas larger molecules (600 to 1,000 Da) cross more slowly.10 Drugs with molecular weights greater than 1,000 Da, such as insulin and heparin, do not cross the placenta in significant amounts. Lipophilic drugs, such as opioids and antibiotics, cross the placenta more easily than do water-soluble drugs. Maternal plasma albumin progressively decreases, while fetal albumin increases during the course of pregnancy, which may result in higher concentrations of certain protein-bound drugs in the fetus. Fetal pH is slightly more acidic than maternal pH, permitting weak bases to more easily cross the placenta. Once in the fetal circulation, the molecule becomes more ionized and less likely to diffuse back into the maternal circulation.10
DRUG SELECTION DURING PREGNANCY
![]() Many misconceptions exist regarding the association of medications and birth defects. Although some drugs have the potential to cause teratogenic effects, most medications required by pregnant women can be used safely.
Many misconceptions exist regarding the association of medications and birth defects. Although some drugs have the potential to cause teratogenic effects, most medications required by pregnant women can be used safely.
The baseline risk for congenital malformations is approximately 3% to 6%, with approximately 3% considered severe.2 Medication exposure is estimated to account for less than 1% of all birth defects. Genetic causes are responsible for 15% to 25%, other environmental issues (e.g., maternal conditions and infections) account for 10%, and the remaining 65% to 75% of congenital malformations result from unknown causes.2
Factors such as the stage of pregnancy during exposure, route of administration, and dose can affect outcomes.2,11 In the first 2 weeks following conception, exposure to a teratogen may result in an “all-or-nothing” effect, which could either destroy the embryo or cause no problems.11 During organogenesis (18 to 60 days post-conception), organ systems are developing, and teratogenic exposures may result in structural anomalies. For the remainder of the pregnancy, exposure to teratogens may result in growth retardation, CNS abnormalities, or death. Examples of medications associated with teratogenic effects in the period of organogenesis include chemotherapy drugs (e.g., methotrexate, cyclophosphamide), sex hormones (e.g., diethylstilbestrol), lithium, retinoids, thalidomide, certain antiepileptic drugs, and coumarin derivatives. Other medications such as non-steroidal antiinflammatory drugs (NSAIDs) and tetracycline derivatives are more likely to exhibit effects in the second or third trimester.
Medications are necessary during pregnancy for treatment of acute and chronic conditions. Identifying patterns of medication use before conception, eliminating nonessential medications and discouraging self-medication, minimizing exposure to medications known to be harmful, and adjusting medication doses are all strategies to optimize the health of the mother while minimizing the risk to the fetus. In summary, a small number of medications have the potential to cause congenital malformations, and many can be avoided during pregnancy. In situations where a drug may be teratogenic but is necessary for maternal care, considerations related to route of administration, dosage form, and dosing may lessen the risk.
Methods and Resources for Determining Drug Safety in Pregnancy
![]() When assessing the safety of using medications during pregnancy, evaluation of the quality of the evidence is important. Ideally, safety data from randomized, controlled trials are most desirable, but pregnant women are not usually eligible for participation in clinical trials. Other types of data commonly used to estimate the risk associated with medication use during pregnancy include animal studies, case reports, case–control studies, prospective cohort studies, historical cohort studies, and voluntary reporting systems.
When assessing the safety of using medications during pregnancy, evaluation of the quality of the evidence is important. Ideally, safety data from randomized, controlled trials are most desirable, but pregnant women are not usually eligible for participation in clinical trials. Other types of data commonly used to estimate the risk associated with medication use during pregnancy include animal studies, case reports, case–control studies, prospective cohort studies, historical cohort studies, and voluntary reporting systems.
Animal studies are a required component of drug testing, but extrapolation of the results to humans is not always valid.12 Thalidomide was found to be safe in some animal models, but proved to have teratogenic effects in human offspring. The value of case reports is limited because birth defects in the offspring of women who used medication during pregnancy may occur by chance.12 Case–control studies identify an outcome (congenital anomaly), match subjects with and without that outcome, and report how often exposure to a suspected agent occurred. Recall bias is a concern, as women with an affected pregnancy may be more likely to remember drugs used during the pregnancy than those with a normal outcome.
Cohort studies evaluate the intervention (use of a particular drug) in a group of persons and compare outcomes in a similar group of subjects without the intervention.12,13 Prospective studies eliminate some of the problems with recall bias, but require time and large numbers of participants. Despite these disadvantages, cohort studies are often used for evaluating the effects of a drug exposure on pregnancy outcomes.
Teratology information services provide pregnant women with information about potential exposures during pregnancy and follow these women throughout the pregnancy to assess the outcomes of the pregnancy.12 Services may publish pooled data to facilitate information sharing about medications used during pregnancy. Some pharmaceutical companies have organized voluntary reporting systems (also called pregnancy registries) for drugs used during pregnancy.
![]() Computerized databases (e.g., www.motherisk.org, LactMed [www.toxnet.nlm.nih.gov]), tertiary compendia, and textbooks with information from large cohorts of treated women offer valuable assistance. New information regarding drug use in pregnancy and lactation can be obtained from searches of the primary literature for cohort and case–control studies.
Computerized databases (e.g., www.motherisk.org, LactMed [www.toxnet.nlm.nih.gov]), tertiary compendia, and textbooks with information from large cohorts of treated women offer valuable assistance. New information regarding drug use in pregnancy and lactation can be obtained from searches of the primary literature for cohort and case–control studies.
The FDA developed risk categories (i.e., A, B, C, D, X, with A considered safe and X considered teratogenic) to guide clinicians regarding medication risk during pregnancy. The FDA ranks very few drugs as safe during pregnancy (category A) because a controlled trial is required to establish safety; this implies that few drugs are safe. Because of multiple limitations of the risk categories, the FDA proposed a new system in 2008 to replace the risk categories with a fetal risk summary and lactation risk summary. Each section discusses clinical considerations and summarizes available data.14
In summary, determining drug safety during pregnancy is limited by the quality of data and the types of study designs that can be used. While information from product labeling may provide a rough estimate of risks for medication-related adverse fetal outcomes, careful evaluation of other available information sources is necessary to make decisions about medication use in pregnant women.
PRECONCEPTION PLANNING
Pregnancy outcomes are influenced by maternal health status, lifestyle, and history prior to conception. The goal of preconception care is health promotion, evidence-based screening, and intervention in all women of reproductive age to ensure optimal health and improve pregnancy outcomes.15 More than 60% of pregnancies in the United States are unintended. Of women who receive prenatal care, 18% seek it after the first trimester.16 Preconception planning is important, since some behaviors and exposures impart risk to the fetus during the first trimester, often before prenatal care is begun or even before pregnancy is detected.15 Table 61-1 lists selected preconception risk factors, the potential adverse pregnancy outcomes, and management or prevention options.
TABLE 61-1 Selected Preconception Risk Factors for Adverse Pregnancy Outcomes
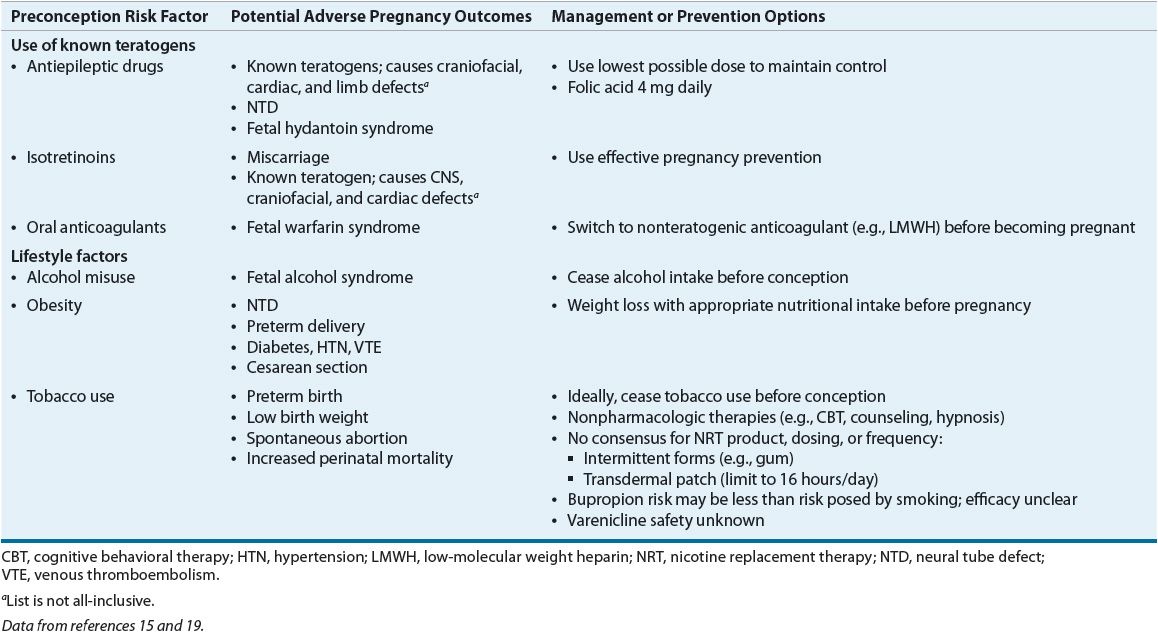
The most common major congenital abnormalities are neural tube defects (NTDs), cleft palate and lip, and cardiac anomalies. Each year in the United States approximately 1 in 1,000 infants are born with NTDs.17 Folic acid supplementation of women substantially reduces the incidence of NTDs in their offspring. This is also true in women who have previously delivered babies with NTDs.16,17 NTDs occur within the first month of conception because neural tube closure occurs during the first month of pregnancy. Folic acid supplementation with between 0.4 and 0.9 mg daily is recommended throughout a woman’s reproductive years, since many pregnancies are unplanned and may not be recognized until after the first month.
Use of alcohol and recreational drugs during pregnancy is associated with birth defects.15 Of births in the United States in 2003, 10% were to mothers who smoked tobacco during pregnancy.15 Smoking can cause preterm birth, low birth weight, and other adverse outcomes. In a systematic review of 72 trials of smoking cessation and perinatal outcomes, incidences of low birth weight and preterm birth were reduced, and birth weight increased by 54 g with smoking cessation.18 Use of nicotine replacement during pregnancy is controversial, since its use is not supported by clinical trial data; however, nicotine replacement theoretically imparts less risk than exposure to the over 4,000 chemicals found in cigarettes.19
Clinical Controversy…
PREGNANCY-INFLUENCED ISSUES
Pregnancy causes or exacerbates conditions that pregnant women commonly experience, including constipation, gastroesophageal reflux, hemorrhoids, and nausea and vomiting. Women with pregnancy-influenced GI issues can be treated safely with lifestyle modification or medications, many of them nonprescription. Gestational diabetes, gestational hypertension, and venous thromboembolism (VTE) have the potential to cause adverse pregnancy consequences. Gestational thyrotoxicosis (GTT) is usually self-limiting.
GI Tract
![]() Constipation during pregnancy is prevalent, affecting 25% to 40% of women and may contribute to the development or exacerbation of hemorrhoids; hemorrhoids are more prevalent in pregnant women compared with the general population.20,21 Light physical exercise and increased intake of dietary fiber and fluid should be instituted first for constipation.21 If additional treatment is needed, supplemental fiber and/or a stool softener is appropriate.22 Osmotic laxatives (polyethylene glycol, lactulose, sorbitol, and magnesium and sodium salts) are acceptable for short-term, intermittent use. Some consider polyethylene glycol the ideal laxative for use in pregnancy.21,22 Senna and bisacodyl can be used occasionally. Castor oil and mineral oil should be avoided because they cause stimulation of uterine contractions and impairment of maternal fat-soluble vitamin absorption, respectively. Data supporting other management options for hemorrhoids during pregnancy are limited. Conservative treatment (i.e., high dietary fiber intake, adequate oral fluid intake, and use of sitz baths) should be tried first. Laxatives and stool softeners can be used if conservative management is inadequate for preventing or treating constipation. Topical anesthetics, skin protectants, and astringents (e.g., witch hazel) can be used for anal irritation and pain. Hydrocortisone may reduce inflammation and pruritis.20
Constipation during pregnancy is prevalent, affecting 25% to 40% of women and may contribute to the development or exacerbation of hemorrhoids; hemorrhoids are more prevalent in pregnant women compared with the general population.20,21 Light physical exercise and increased intake of dietary fiber and fluid should be instituted first for constipation.21 If additional treatment is needed, supplemental fiber and/or a stool softener is appropriate.22 Osmotic laxatives (polyethylene glycol, lactulose, sorbitol, and magnesium and sodium salts) are acceptable for short-term, intermittent use. Some consider polyethylene glycol the ideal laxative for use in pregnancy.21,22 Senna and bisacodyl can be used occasionally. Castor oil and mineral oil should be avoided because they cause stimulation of uterine contractions and impairment of maternal fat-soluble vitamin absorption, respectively. Data supporting other management options for hemorrhoids during pregnancy are limited. Conservative treatment (i.e., high dietary fiber intake, adequate oral fluid intake, and use of sitz baths) should be tried first. Laxatives and stool softeners can be used if conservative management is inadequate for preventing or treating constipation. Topical anesthetics, skin protectants, and astringents (e.g., witch hazel) can be used for anal irritation and pain. Hydrocortisone may reduce inflammation and pruritis.20
Between 40% and 80% of pregnant women experience gastroesophageal reflux disease.21 An algorithm starting with lifestyle and dietary modifications (e.g., small, frequent meals; alcohol and tobacco avoidance; food avoidance before bedtime; elevation of the head of the bed) should be used. If symptoms are not relieved, antacids (aluminum, calcium, or magnesium preparations) or sucralfate are acceptable; however, sodium bicarbonate and magnesium trisilicate should be avoided. Histamine-2 (H2) receptor blockers can be used for patients unresponsive to lifestyle changes and antacids; evidence supports the use of ranitidine and cimetidine. Literature evaluating the use of famotidine and nizatidine is limited, but they are likely safe. Less data are available regarding the use of proton pump inhibitors (PPIs) during pregnancy. Although a recent cohort study of 5,082 live births with first trimester exposure to PPIs found no increased risk of major birth defects,23 use of PPIs should be reserved for women who do not respond to H2 antagonists.
Nausea and vomiting of pregnancy (NVP) is estimated to affect up to 90% of pregnant women. NVP usually begins during the fifth week of gestation and lasts through week 20; peak symptoms occur between weeks 10 and 16.21,24 Hyperemesis gravidarum (HG; i.e., unrelenting vomiting causing weight loss of more than 5% prepregnancy weight, dehydration, electrolyte imbalance, and ketonuria) occurs in 0.3% to 2.3% of women.25 Dietary modifications, such as eating frequent, small, bland meals and avoiding fatty foods, may be helpful. Applying pressure at acupressure point P6 on the volar aspect of the wrist may be beneficial. Pharmacotherapeutic approaches for NVP that have shown efficacy include pyridoxine (vitamin B6), and antihistamines (including doxylamine).24 Phenothiazines and metoclopramide are generally considered safe, but sedation and extrapyramidal effects, including dystonia, may limit use.24 Increasing evidence of safety and efficacy with ondansetron is emerging; ondansetron is better tolerated than older antiemetics.24 Corticosteroids are effective for HG but are associated with a small increase in the risk of oral clefts when used during the first trimester. Ginger has shown efficacy for hyperemesis in randomized, controlled trials and is probably safe.21,24
Gestational Diabetes
![]() Gestational diabetes mellitus (GDM) is glucose intolerance of any degree identified during pregnancy, either of new onset or first recognition. It develops in about 7% of pregnant women, although the prevalence ranges from 1% to 14%.26,27 Risks of GDM are many and include fetal loss, increased risk of major malformations, and fetal macrosomia. Although the U.S. Preventative Services Task Force Independent Expert Panel found a lack of evidence proving that screening for gestational diabetes decreases adverse maternal and fetal outcomes, a consensus panel of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommends universal screening of pregnant women not previously diagnosed with diabetes.26–29 At the first prenatal visit, all women considered high-risk for diabetes (e.g., obesity, glycosuria, strong family history of diabetes) should be screened for overt diabetes using either the A1C, fasting plasma glucose (FPG), or random plasma glucose (RPG).27,29 Overt diabetes occurs if the A1C is greater than or equal to 6.5% (0.065; 48 mmol/mol Hgb), FPG is greater than or equal to 126 mg/dL (7.0 mmol/L), or RPG is greater than or equal to 200 mg/dL (11.1 mmol/L; requires confirmation with A1C or FPG). If overt diabetes is not diagnosed or for women not at high-risk for diabetes, the IADPSG recommends screening for GDM at weeks 24 to 28 using a 75-g oral glucose tolerance test (OGTT).27,29 The American College of Obstetricians and Gynecologists (ACOG) recommends screening for gestational diabetes based on clinical risk factors or with the use of a 50 g, 1-hour glucose challenge test followed by a 100 g, 3-hour OGTT to diagnose GDM; this is commonly referred to as the “two-step” method.30 Screening and diagnosis of GDM using the OGTT is described in the American Diabetes Association practice guidelines.26,27
Gestational diabetes mellitus (GDM) is glucose intolerance of any degree identified during pregnancy, either of new onset or first recognition. It develops in about 7% of pregnant women, although the prevalence ranges from 1% to 14%.26,27 Risks of GDM are many and include fetal loss, increased risk of major malformations, and fetal macrosomia. Although the U.S. Preventative Services Task Force Independent Expert Panel found a lack of evidence proving that screening for gestational diabetes decreases adverse maternal and fetal outcomes, a consensus panel of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommends universal screening of pregnant women not previously diagnosed with diabetes.26–29 At the first prenatal visit, all women considered high-risk for diabetes (e.g., obesity, glycosuria, strong family history of diabetes) should be screened for overt diabetes using either the A1C, fasting plasma glucose (FPG), or random plasma glucose (RPG).27,29 Overt diabetes occurs if the A1C is greater than or equal to 6.5% (0.065; 48 mmol/mol Hgb), FPG is greater than or equal to 126 mg/dL (7.0 mmol/L), or RPG is greater than or equal to 200 mg/dL (11.1 mmol/L; requires confirmation with A1C or FPG). If overt diabetes is not diagnosed or for women not at high-risk for diabetes, the IADPSG recommends screening for GDM at weeks 24 to 28 using a 75-g oral glucose tolerance test (OGTT).27,29 The American College of Obstetricians and Gynecologists (ACOG) recommends screening for gestational diabetes based on clinical risk factors or with the use of a 50 g, 1-hour glucose challenge test followed by a 100 g, 3-hour OGTT to diagnose GDM; this is commonly referred to as the “two-step” method.30 Screening and diagnosis of GDM using the OGTT is described in the American Diabetes Association practice guidelines.26,27
Clinical Controversy…
Dietary modification is considered first-line therapy for all women who have GDM, with additional caloric restriction for obese women.31 Daily self-monitoring of blood glucose is required. Drug therapy should be initiated if the following levels are not achieved with dietary modification: FPG concentrations below 90 to 99 mg/dL (5.0 to 5.5 mmol/L), 1-hour postprandial plasma glucose concentration less than or equal to 140 mg/dL (7.8 mmol/L), or 2-hour postprandial plasma glucose concentration below 120 to 127 mg/dL (6.7 to 7.0 mmol/L).31,32 Traditionally, insulin has been the drug of choice for diabetes management during pregnancy because it does not cross the placenta. Glyburide is an alternative because it minimally crosses the placenta. Increasing data suggest that, although it crosses the placenta, metformin appears to lack teratogenicity making it another alternative to insulin.31,32
Evidence supporting dietary modification, self-monitored blood glucose, exercise, and pharmacologic interventions for women with GDM is largely based on one randomized clinical trial that showed reductions in perinatal morbidity (composite of death, nerve palsy, bone fracture, and shoulder dystocia) with nutritional education, blood glucose monitoring, and insulin treatment.33,34
Hypertensive Disorders of Pregnancy
![]() Hypertensive disorders of pregnancy (HDP) complicate approximately 10% of pregnancies. Four categories of HDP are established: chronic hypertension (preexisting hypertension or developing before 20 weeks’ gestation), gestational hypertension (hypertension without proteinuria developing after 20 weeks’ gestation), preeclampsia (hypertension with proteinuria), and preeclampsia superimposed on chronic hypertension.35 Hypertension in pregnancy is defined as a diastolic blood pressure (dBP) 90 mm Hg or greater based upon the average of two or more measurements from the same arm.36 Nondrug managements of HDP center on activity restriction, stress reduction, and exercise; however, no evidence indicates that any of these approaches improves pregnancy outcome, and prolonged bed rest may increase the risk of venous thromboembolic disease.36 Use of supplemental calcium 1 to 2 g/day decreases the relative risk of hypertension by 30% (range, 14% to 43%) and preeclampsia by 48% (range, 31% to 67%).37 High-risk patients (those with the lowest initial calcium intake) benefited most; however, even women with adequate calcium intake at baseline had a 38% decrease in risk of preeclampsia. Therefore, 1 g/day of supplemental calcium is appropriate for all pregnant women. Antihypertensive drug therapy is discussed under Chronic Illnesses in Pregnancy.
Hypertensive disorders of pregnancy (HDP) complicate approximately 10% of pregnancies. Four categories of HDP are established: chronic hypertension (preexisting hypertension or developing before 20 weeks’ gestation), gestational hypertension (hypertension without proteinuria developing after 20 weeks’ gestation), preeclampsia (hypertension with proteinuria), and preeclampsia superimposed on chronic hypertension.35 Hypertension in pregnancy is defined as a diastolic blood pressure (dBP) 90 mm Hg or greater based upon the average of two or more measurements from the same arm.36 Nondrug managements of HDP center on activity restriction, stress reduction, and exercise; however, no evidence indicates that any of these approaches improves pregnancy outcome, and prolonged bed rest may increase the risk of venous thromboembolic disease.36 Use of supplemental calcium 1 to 2 g/day decreases the relative risk of hypertension by 30% (range, 14% to 43%) and preeclampsia by 48% (range, 31% to 67%).37 High-risk patients (those with the lowest initial calcium intake) benefited most; however, even women with adequate calcium intake at baseline had a 38% decrease in risk of preeclampsia. Therefore, 1 g/day of supplemental calcium is appropriate for all pregnant women. Antihypertensive drug therapy is discussed under Chronic Illnesses in Pregnancy.
While preeclampsia usually develops after 20 weeks’ gestation, up to 30% of chronic and gestational hypertension are complicated by preeclampsia. Preeclampsia is a multisystem syndrome that complicates 2% to 8% of pregnancies and can cause poorer outcomes, including renal failure, maternal morbidity/mortality, preterm delivery, and intrauterine growth restriction.38,39 Risk factors for development of preeclampsia include primiparity, previous preeclampsia, prepregnancy body mass index above 30 kg/m2, tobacco use, underlying medical conditions (e.g., diabetes, antiphospholipid antibodies, autoimmune disease, renal disease), multiple gestations (i.e., twins), and ethnicity (black greater than white or Hispanic). Maternal age over 40 years is also a potential risk factor.39 Signs and symptoms of preeclampsia include blood pressure elevation; proteinuria (300 [or more] mg/24 h); persistent severe headache; persistent new epigastric pain; visual changes; vomiting; hyperreflexia; sudden and severe swelling of hands, face, or feet; HELLP (hemolysis, elevated liver enzymes, low platelets); and increased serum creatinine. Low-dose aspirin (75 to 81 mg/day) in women at risk for preeclampsia decreases the risk of its development by 17%, which corresponds to prevention of one preeclampsia case for every 72 at-risk women treated. Decreased rates of preterm birth (8% reduction) and fetal or neonatal death (14% reduction) also result from low-dose aspirin use.40 Treatment of hypertension in women with preeclampsia depends upon the blood pressure measurement and follows the same principles discussed under Chronic Illnesses in Pregnancy. The only cure for preeclampsia is delivery of the placenta.41
Preeclampsia may progress rapidly to eclampsia, which is the occurrence of seizures superimposed on preeclampsia. Eclampsia is a medical emergency. In high-risk women (i.e., previous severe preeclampsia, renal disease, autoimmune disease, diabetes, and chronic hypertension), use of low-dose aspirin prevents one case of preeclampsia for every 19 women treated.40 Magnesium sulfate decreases the risk of progression to eclampsia by almost 60%; it is recommended to prevent eclampsia as well as treat eclamptic seizures. The usual dose for magnesium sulfate is 4 to 6 g IV over 15 to 20 minutes followed by a 2 g/h continuous infusion; duration of use varies, but the usual duration is 24 hours. Diazepam and phenytoin should be avoided.42
Thyroid Abnormalities
![]() During pregnancy, stimulation of the thyroid gland may occur because of hCG’s structural similarity to thyroid-stimulating hormone (TSH; thyrotropin).43 In 1% to 3% of pregnancies, gestational transient thyrotoxicosis (GTT) may result. Occurrence of GTT is often associated with HG. By 20 weeks’ gestation, GTT usually resolves as production of hCG declines. Treatment with antithyroid medication is not usually needed.43 Nausea and vomiting can be treated as for patients without this pseudohyperthyroid state.
During pregnancy, stimulation of the thyroid gland may occur because of hCG’s structural similarity to thyroid-stimulating hormone (TSH; thyrotropin).43 In 1% to 3% of pregnancies, gestational transient thyrotoxicosis (GTT) may result. Occurrence of GTT is often associated with HG. By 20 weeks’ gestation, GTT usually resolves as production of hCG declines. Treatment with antithyroid medication is not usually needed.43 Nausea and vomiting can be treated as for patients without this pseudohyperthyroid state.
Although not all women experience postpartum thyroiditis (PPT) similarly, the typical presentation is characterized by transient hyperthyroidism during the first 6 months postpartum, a period of transient hypothyroidism, and, finally, euthyroidism within 1 year.43 The initial hyperthyroid state usually does not require treatment; however, β-blockers (propranolol, starting at 10 to 20 mg daily as needed) can provide symptomatic relief of adrenergic symptoms. Because PTT is from a destructive inflammation process and not overproduction of thyroid hormone, antithyroid drugs are ineffective. Levothyroxine is recommended in the hypothyroid phase of PPT for severe hypothyroid symptoms, duration of hypothyroidism greater than 6 months, breast-feeding women, or if another pregnancy is attempted. Levothyroxine replacement is suggested for a total of 6 to 12 months.43 Occurrence of permanent hypothyroidism ranges from 2% to 21% of women affected by PPT.
Thromboembolism
![]() The risk of VTE in pregnant women is increased by five- to tenfold over non-pregnant women.44 Low-molecular-weight heparin (LMWH) is recommended over unfractionated heparin (UFH) and warfarin for treatment of acute thromboembolism during pregnancy. Treatment should be continued throughout pregnancy and for 6 weeks after delivery; the minimum total duration of therapy should not be less than 3 months. Fondaparinux and injectable direct thrombin inhibitors (e.g., lepirudin, bivalirudin) should be avoided unless a severe allergy to heparin (e.g., heparin-induced thrombocytopenia) is present. Dabigatran, rivaroxaban, and apixaban are not recommended.44 Warfarin is not used because it causes nasal hypoplasia, stippled epiphyses, limb hypoplasia, and eye abnormalities; the risk period appears to be between 6 and 12 weeks’ gestation. CNS anomalies are associated with second- and third-trimester exposure.
The risk of VTE in pregnant women is increased by five- to tenfold over non-pregnant women.44 Low-molecular-weight heparin (LMWH) is recommended over unfractionated heparin (UFH) and warfarin for treatment of acute thromboembolism during pregnancy. Treatment should be continued throughout pregnancy and for 6 weeks after delivery; the minimum total duration of therapy should not be less than 3 months. Fondaparinux and injectable direct thrombin inhibitors (e.g., lepirudin, bivalirudin) should be avoided unless a severe allergy to heparin (e.g., heparin-induced thrombocytopenia) is present. Dabigatran, rivaroxaban, and apixaban are not recommended.44 Warfarin is not used because it causes nasal hypoplasia, stippled epiphyses, limb hypoplasia, and eye abnormalities; the risk period appears to be between 6 and 12 weeks’ gestation. CNS anomalies are associated with second- and third-trimester exposure.
Recurrent VTE is divided into three categories: low risk, intermediate risk, and high risk of recurrence. Antepartum monitoring is recommended for women with a single episode of VTE who have a low risk of recurrence (i.e., one transient risk factor [e.g., surgery, injury, lengthy travel, or immobility]). For intermediate risk (i.e., hormone-related, pregnancy-related, or unprovoked VTE) and high risk (i.e., more than one unprovoked VTE or continuous risk factors), antipartum prophylaxis with LMWH plus 6-week postpartum prophylaxis with either LMWH or warfarin is recommended. Specific recommendations for thrombophilias (e.g., antiphospholipid antibodies, Factor V Leiden, protein C and S deficiencies) can be found in the American College of Chest Physicians clinical practice guidelines.44
Women with prosthetic heart valves should receive LMWH (twice daily) or UFH (every 12 hours) during pregnancy. LMWH should be adjusted to achieve a peak anti-Xa level at 4 hours postsubcutaneous dose, while UFH treatment should target a midinterval aPTT at least twice the control value or an anti-Xa heparin level of 0.35 to 0.7 units/mL.44 After a discussion of potential risks, LMWH or UFH can be used until week 13 of gestation with subsequent substitution of warfarin until the middle of the third trimester when LMWH or UFH should be resumed. In women considered very high-risk for VTE (e.g., older-generation prosthetic mitral valve, history of thromboembolism), prevention of maternal complications such as valve thrombosis exceeds the risk of fetal malformation; use of warfarin is appropriate until replacement with LMWH or UFH near the end of the third trimester. High-risk women with prosthetic heart valves may also receive low-dose aspirin (75 to 100 mg/day).44
ACUTE CARE ISSUES IN PREGNANCY
In some cases, the risks associated with the acute illness are magnified during pregnancy, and early screening and treatment become critical. In other cases, such as during treatment of certain sexually transmitted diseases, the urgency regarding treatment comes from an increased likelihood of infection leading to preterm labor. Occasionally, common acute care issues, such as migraine headache, improve during pregnancy.
Urinary Tract Infection
![]() The most common infections in pregnant and nonpregnant women are urinary tract infections (UTIs). Typically, UTIs are characterized as asymptomatic (e.g., asymptomatic bacteriuria) or symptomatic (e.g., lower [cystitis] or upper [pyelonephritis]).45,46 Escherichia coli is the primary cause of infection in 75% to 90% of cases.46 Other gram-negative rods, such as Proteus and Klebsiella, as well as Group B Streptococcus (GBS) account for some infections. The presence of GBS in the urine indicates heavy colonization of the genitourinary tract, increasing the risk for GBS infection in the newborn.47
The most common infections in pregnant and nonpregnant women are urinary tract infections (UTIs). Typically, UTIs are characterized as asymptomatic (e.g., asymptomatic bacteriuria) or symptomatic (e.g., lower [cystitis] or upper [pyelonephritis]).45,46 Escherichia coli is the primary cause of infection in 75% to 90% of cases.46 Other gram-negative rods, such as Proteus and Klebsiella, as well as Group B Streptococcus (GBS) account for some infections. The presence of GBS in the urine indicates heavy colonization of the genitourinary tract, increasing the risk for GBS infection in the newborn.47
The incidence of asymptomatic bacteriuria ranges from 2% to 10%. Untreated, bacteriuria progresses to pyelonephritis in approximately 30% of pregnant women.46,47 While no consensus regarding screening for asymptomatic bacteriuria exists, a urine culture obtained at the first prenatal visit is appropriate; some advocate a urine culture in each trimester. Use of rapid screening tests, such as dipsticks, should be avoided because of poor performance in pregnant women.47 Acute cystitis occurs in about 1% to 3% of pregnant women. Signs and symptoms of acute cystitis include urgency, frequency, hematuria, pyuria, and dysuria.46,47
Treatment of asymptomatic bacteriuria is necessary to prevent pyelonephritis. For asymptomatic bacteriuria, the agents of choice and treatment duration are not well defined. Treatment of acute cystitis is similar to that of asymptomatic bacteriuria. Using outcomes of cure rates, recurrent infection, incidence of preterm delivery or rupture of membranes, admission to neonatal intensive care, need for change of antibiotic, or incidence of prolonged fever, antibiotic treatment has demonstrated effectiveness in treating symptomatic UTIs (including pyelonephritis) in pregnancy. No specific treatment appeared superior to other commonly used treatments.45,47 Treatment courses for asymptomatic bacteriuria and cystitis of 7 to 14 days are common, but shorter courses of therapy may be sufficient.
The most commonly used antibiotics to treat asymptomatic bacteriuria and cystitis are the β-lactams (including penicillins and cephalosporins) and nitrofurantoin.45,47 β-Lactams are not known teratogens; however, the incidence of E. coli resistance to ampicillin and amoxicillin limits their use as single agents. Nitrofurantoin is not active against Proteus species and should not be used after week 37 in patients with glucose-6-phosphate dehydrogenase deficiency because of a theoretical risk for hemolytic anemia in the neonate. Sulfa-containing drugs can contribute to the development of newborn kernicterus; use should be avoided during the last weeks of gestation. Trimethoprim is a folate antagonist and is relatively contraindicated during the first trimester because of associations with cardiovascular malformations. Regionally, increased rates of E. coli resistance to trimethoprim-sulfa may limit its use. Fluoroquinolones and tetracyclines are contraindicated because of potential associations with impaired cartilage development and deciduous teeth discoloration (if given after 5 months’ gestation), respectively.47
Patients with pyelonephritis usually present with bacteriuria and systemic symptoms of costovertebral angle tenderness, dysuria, fever, flank pain, nausea, and vomiting.46,47 Complications of pyelonephritis include premature delivery, low infant birth weight, hypertension, anemia, bacteremia, and transient renal failure. Hospitalization is the standard of care for pregnant women.47 Inpatient therapy has included parenteral administration of cephalosporins (e.g., cefazolin, ceftriaxone), ampicillin plus gentamicin, or ampicillin–sulbactam. Switching to oral antibiotics can occur after the woman is afebrile for 48 hours; however, nitrofurantoin should be avoided because it does not achieve therapeutic levels outside of the urine. Outpatient antibiotic therapy can be considered after initial inpatient observation in women who are afebrile and less than 24 weeks’ gestation. The total duration of antibiotic therapy for acute pyelonephritis is 10 to 14 days.47 Suppression therapy with nitrofurantoin can be considered for the remainder of gestation.46
Sexually Transmitted Infections
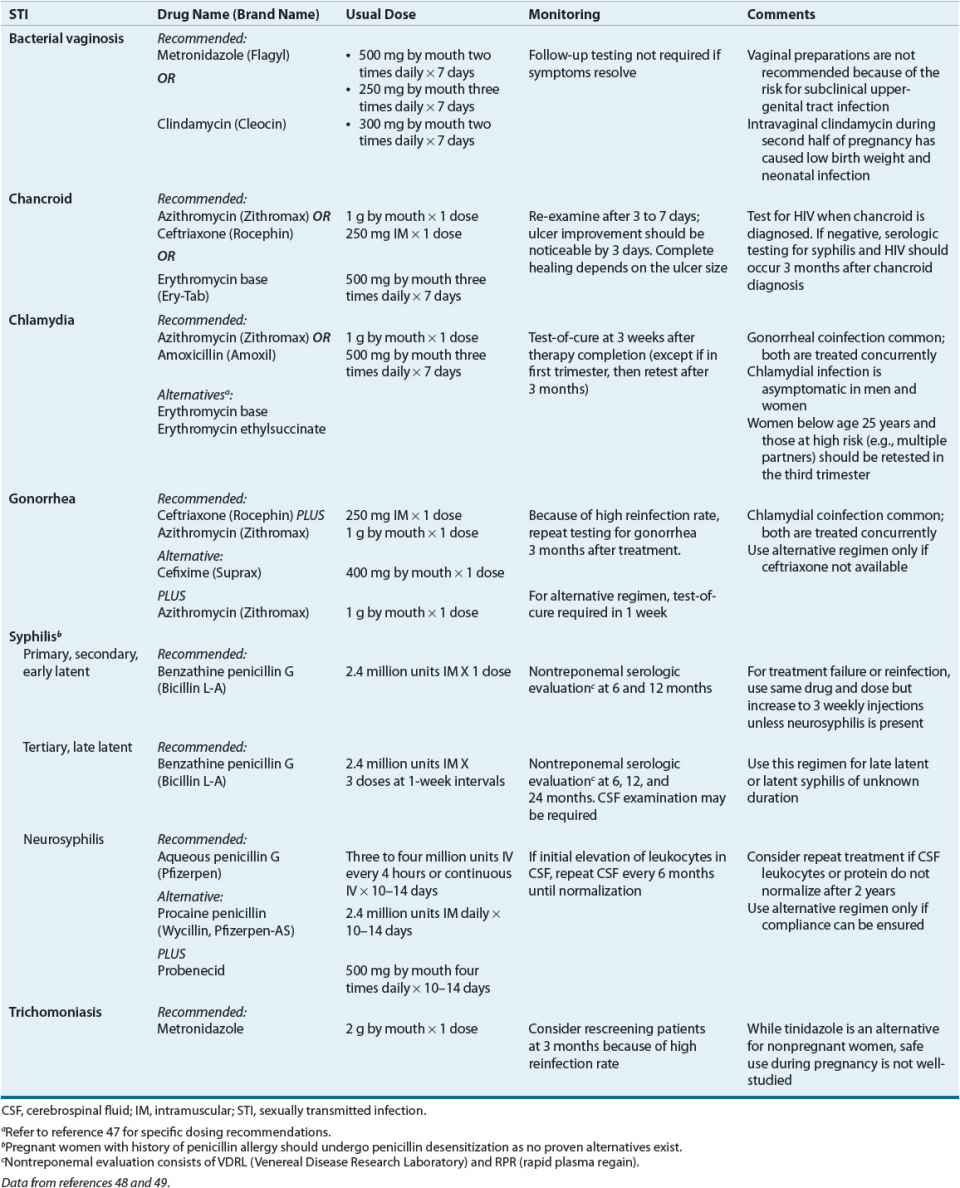
![]() Sexually transmitted infections (STIs) in pregnant women range from infections that may be transmitted across the placenta and infect the infant prenatally (e.g., syphilis) to organisms that may be transmitted during birth and cause neonatal infection (e.g., Chlamydia trachomatis, Neisseria gonorrhoeae, or herpes simplex virus [HSV]) to infections that pose a threat for preterm labor (e.g., bacterial vaginosis [BV]). Initial screening during the first prenatal visit is recommended for HIV, C. trachomatis, and syphilis. Women at high risk for gonorrhea or who live in an area of high prevalence as well as women at high risk for hepatitis C should also be screened during the first prenatal visit. Screening for hepatitis B using the surface antigen should occur during the first trimester. Treatment for selected STIs is summarized in Table 61-2.
Sexually transmitted infections (STIs) in pregnant women range from infections that may be transmitted across the placenta and infect the infant prenatally (e.g., syphilis) to organisms that may be transmitted during birth and cause neonatal infection (e.g., Chlamydia trachomatis, Neisseria gonorrhoeae, or herpes simplex virus [HSV]) to infections that pose a threat for preterm labor (e.g., bacterial vaginosis [BV]). Initial screening during the first prenatal visit is recommended for HIV, C. trachomatis, and syphilis. Women at high risk for gonorrhea or who live in an area of high prevalence as well as women at high risk for hepatitis C should also be screened during the first prenatal visit. Screening for hepatitis B using the surface antigen should occur during the first trimester. Treatment for selected STIs is summarized in Table 61-2.
TABLE 61-2 Management of Sexually Transmitted Diseases in Pregnancy