There are depolarizing and nondepolarizing NMBAs. Depolarizing NMBAs physically resemble acetylcholine and, therefore, bind and activate acetylcholine receptors. Succinylcholine is currently the only available depolarizing NMBA and is not used for long-term use in ICUs.
Nondepolarizing NMBAs also bind acetylcholine receptors but do not activate them—they are competitive antagonists. The difference in the mechanism of action also accounts for different effects in certain diseases. If there is a long-term decrease in acetylcholine release, the number of acetylcholine receptors within the muscle increases. This upregulation causes an increased response to depolarizing NMBAs but a resistance to nondepolarizing NMBAs (i.e., more receptors must be blocked). Conditions in which there are fewer acetylcholine receptors (e.g., myasthenia gravis) lead to an increase in sensitivity to nondepolarizing NMBAs.
Adult skeletal muscle retains an ability to synthesize both the mature adult nAChR as well as an immature nAChR variant in which a gamma subunit is substituted for the normal epsilon subunit. Synthesis of immature (fetal) receptors may be triggered in the presence of certain diseases (e.g., Guillain-Barré syndrome, stroke) and other conditions producing loss of nerve function. These immature nAChRs are distinguished by three features. First, immature receptors are not localized to the muscle endplate but migrate across the entire membrane surface.2 Second, the immature receptors are metabolically short-lived (<24 hours) and more ionically active, having a 2- to 10-fold longer channel “open time.” Lastly, these immature receptors are more sensitive to the depolarizing effects of such drugs as succinylcholine and more resistant to the effects of competitive antagonists, such as pancuronium. This increase in the number of immature acetylcholine receptors may account for the tachyphylaxis seen with NMBAs and some of the complications associated with their use. For the remainder of this document, only nondepolarizing NMBAs will be discussed.
Pharmacology of Neuromuscular-Receptor Blockers
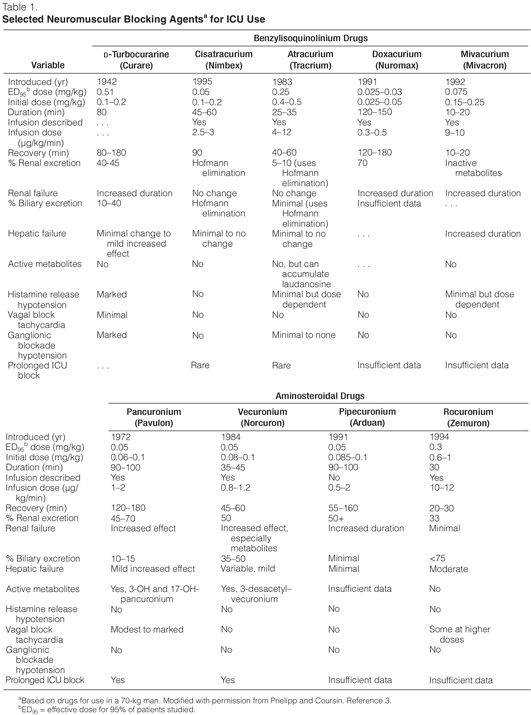
Aminosteroidal Compounds. The aminosteroidal compounds include pancuronium, pipecuronium, vecuronium, and rocuronium (Tables 1 and 2).3–11
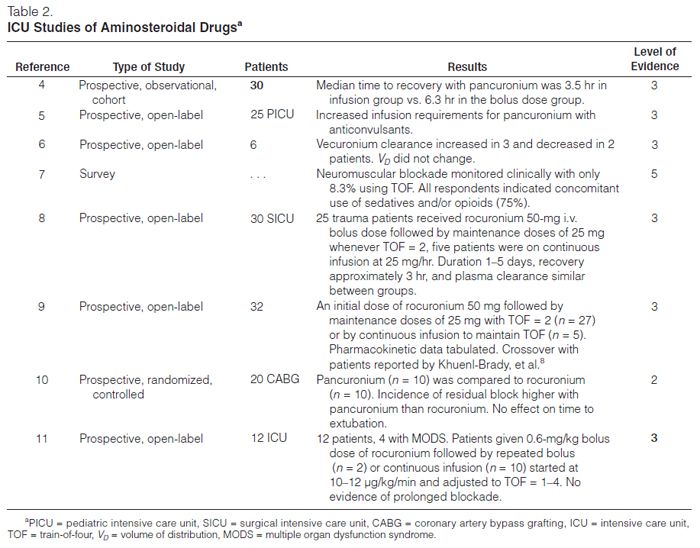
Pancuronium. Pancuronium, one of the original NMBAs used in ICUs, is a long-acting, nondepolarizing compound that is effective after an intravenous bolus dose of 0.06–0.1 mg/kg for up to 90 minutes. Though it is commonly given as an i.v. bolus, it can be used as a continuous infusion12 by adjusting the dose to the degree of neuromuscular blockade that is desired (Table 1). Pancuronium is vagolytic (more than 90% of ICU patients will have an increase in heart rate of ≥10 beats/min), which limits its use in patients who cannot tolerate an increase in heart rate.12 In patients with renal failure or cirrhosis, pancuronium’s neuromuscular blocking effects are prolonged because of its increased elimination half-life and the decreased clearance of its 3-hydroxypancuronium metabolite that has one-third to one-half the activity of pancuronium.
Pipecuronium. Pipecuronium is another long-acting NMBA with an elimination half-life of about two hours, similar to pancuronium’s. Khuenl-Brady and colleagues13 conducted an open-label evaluation of pipecuronium compared with pancuronium in 60 critically ill patients to determine the minimum doses required for ventilatory management. The administration of 8 mg of either drug followed by intermittent boluses of 4–6 mg when needed resulted in optimal paralysis. Patients were paralyzed for a mean duration of 62.6 hours (45–240 hours) and 61.5 hours (46–136 hours) with pancuronium and pipecuronium, respectively. No adverse effects were attributed to either drug. Perhaps because of this lack of difference and because there are no recent studies examining pipecuronium’s use in the ICU, most clinicians continue to use the more familiar drug, pancuronium.
Vecuronium. Vecuronium is an intermediate-acting NMBA that is a structural analogue of pancuronium and is not vagolytic. An i.v. bolus dose of vecuronium 0.08–0.1 mg/kg, produces blockade within 60–90 seconds that typically lasts 25–30 minutes. After an i.v. bolus dose, vecuronium is given as a 0.8–1.2-μg/kg/min continuous infusion, adjusting the rate to the degree of blockade desired. Because up to 35% of a dose is renally excreted, patients with renal failure will have decreased drug requirements. Similarly, because up to 50% of an injected dose is excreted in bile, patients with hepatic insufficiency will also have decreased drug requirements to maintain adequate blockade. The 3-desacetylvecuronium metabolite has 50% of the pharmacologic activity of the parent compound, so patients with organ dysfunction may have increased plasma concentrations of both the parent compound and the active metabolite, which contributes to the prolongation of blockade if the dose is not adjusted. Vecuronium has been reported to be more commonly associated with prolonged blockade once discontinued, compared with other NMBAsa. Members of the task force believe that vecuronium is being used with decreased frequency in the ICU.
Vecuronium has been studied in open-label prospective trials.14,15 In one of these studies, the mean infusion rate for vecuronium was 0.9 ± 0.1 μg/kg/min for a mean duration of 80 ± 7 hours. Recovery of a train-of-four (TOF) ratio of ≥0.7 was significantly longer than with cisatracurium.15 Recovery time averaged 1–2 hours but ranged from ≤30 minutes to more than 48 hours.
Although Rudis, et al.14 observed no difference in the incidence of prolonged blockade between patients receiving vecuronium with and without concomitant administration of corticosteroids, the opinion of the task force was that patients receiving vecuronium and corticosteroids were at increased risk of prolonged weakness once the drug was discontinued.
Rocuronium. Rocuronium is a newer nondepolarizing NMBA with a monoquaternary steroidal chemistry that has an intermediate duration of action and a very rapid onset. When given as a bolus dose of 0.6–1 mg/kg, blockade is almost always achieved within two minutes, with maximum blockade occurring within three minutes. Continuous infusions are begun at 10 μg/kg/min.8 Rocuronium’s metabolite, 17-des-acetylrocuronium, has only 5–10% activity compared with the parent compound.
Sparr, Khuenl-Brady, and colleagues8,9 studied the dose requirements, recovery times, and pharmacokinetics of rocuronium in 32 critically ill patients, 27 of whom were given intermittent bolus doses, and 5 received a continuous infusion. The median duration of drug administration was 29 hours and 63.4 hours in the bolus dose and infusion groups, respectively. The mean dose of rocuronium required to maintain 80% blockade was 0.34 mg/kg, and the median infusion rate required to maintain one twitch of the TOF was 0.54 mg/kg/hr. The median time from the last bolus dose to the appearance of TOF response was 100 minutes; in the infusion group, the TOF response returned 60 minutes after the infusion was stopped.
Rapacuronium. Rapacuronium, a propionate analogue of vecuronium, was marketed as a nonde polarizing NMBA as an alternative to succinylcholine. It was withdrawn from the market on March 27, 2001, because of reports of morbidity (bronchospasm) and mortality associated with its use.
Benzylisoquinolinium Compounds. The benzylisoquinolinium compounds include D-tubocuranine, atracurium, cisatracurium, doxacurium, and mivacurium (Tables 1 and 3).12,15,16,31
D-Tubocurarine. Tubocurarine was the first nonde-polarizing NMBA to gain acceptance and usage in the ICU. This long-acting benzylisoquinolinium agent is rarely used in ICUs because it induces histamine release and autonomic ganglionic blockade. Hypotension is rare, however, when the agent is administered slowly in appropriate dosages (e.g., 0.1–0.2 mg/kg). Metabolism and elimination are affected by both renal and hepatic dysfunction.
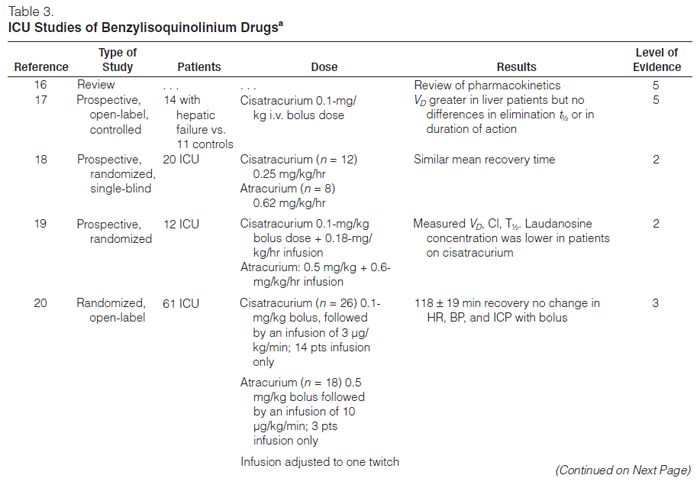
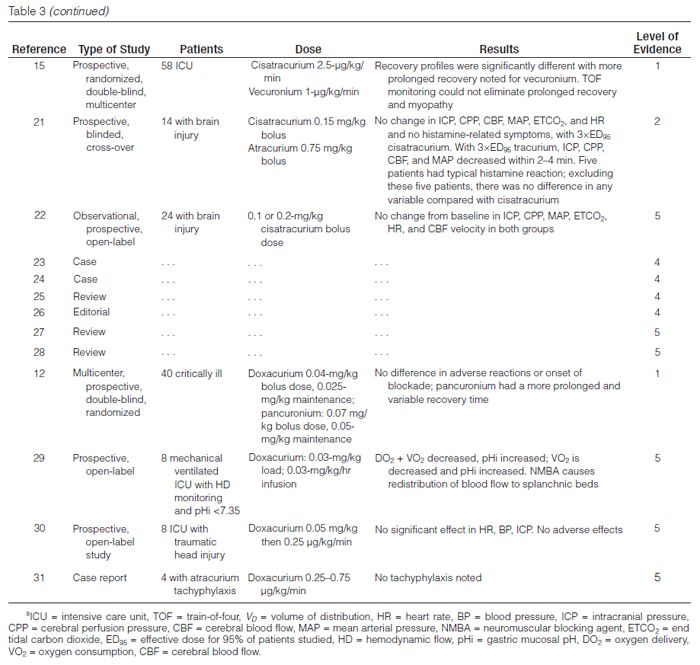
Atracurium. Atracurium is an intermediate-acting NMBA with minimal cardiovascular adverse effects and is associated with histamine release at higher doses. It is inactivated in plas-ma by ester hydrolysis and Hofmann elimination so that renal or hepatic dysfunction does not affect the duration of blockade.
Laudanosine is a breakdown product of Hofmann elimination of atracurium and has been associated with central nervous system excitation. This has led to concern about the possibility of precipitating seizures in patients who have received extremely high doses of atracurium or who are in hepatic failure (laudanosine is metabolized by the liver). There has been only one report of a surgical patient who had a seizure while receiving atracurium.32
Atracurium has been administered to various critically ill patient populations, including those with liver failure,17 brain injury,21 or multiple organ dysfunction syndrome (MODS), to facilitate mechanical ventilation. In these reports, atracurium infusion rates varied widely, but they typically ranged from 10 to 20 μg/kg/min with doses adjusted to clinical endpoints or by TOF monitoring. Infusion durations ranged from ≤24 hours to >200 hours. Recovery of normal neuromuscular activity usually occurred within one to two hours after stopping the infusions and was independent of organ function. Long–term infusions have been associated with the development of tolerance, necessitating significant dose increases or conversion to other NMBAs.31,33 Atracurium has been associated with persistent neuromuscular weakness as have other NMBAs.34–38
Cisatracurium. Cisatracurium, an isomer of atracurium, is an intermediate-acting benzyliso-quinolinium NMBA that is increasingly used in lieu of atracurium. It produces few, if any, cardiovascular effects and has a lesser tendency to produce mast cell degranulation than atracurium. Bolus doses of 0.1–0.2 mg/kg result in paralysis in an average of 2.5 minutes, and recovery begins at approximately 25 minutes; maintenance infusions should be started at 2.5–3 μg/kg/min. Cisatracurium is also metabolized by ester hydrolysis and Hofmann elimination, so the duration of blockade should not be affected by renal or hepatic dysfunction. Prolonged weakness has been reported following the use of cisatracurium.38
Cisatracurium has been compared with atracurium and vecuronium for facilitating mechanical ventilation in several open-label prospective trials.15,18–21 Cisatracurium infusion rates ranged from 2 to 8 μg/kg/min and were adjusted to clinical endpoints or to TOF count. Infusion durations varied from 4 to 145 hours. Recovery of a TOF ratio >0.7 occurred within 34–85 minutes after drug discontinuation and was independent of organ function. These recovery times are similar to those seen with atracurium18,21 and less than those observed with vecuronium.15
Doxacurium. Doxacurium, a long-acting benzyliso-quinolinium agent, is the most potent NMBA currently available. Doxacurium is essentially free of hemodynamic adverse effects. Initial doses of doxacurium 0.05–0.1 mg/kg may be given with maintenance infusions of 0.3–0.5 μg/kg/min and adjusted to the degree of blockade desired. An initial bolus dose lasts an average of 60–80 minutes. Doxacurium is primarily eliminated by renal excretion. In elderly patients and patients with renal dysfunction, a significant prolongation of effect may occur.
Murray and colleagues12 conducted a prospective, randomized, controlled, multicenter comparison of intermittent doses of doxacurium and pancuronium in 40 critically ill patients requiring neuromuscular blockade to optimize mechanical ventilation or to lower intracranial pressure (ICP). Patients were given another bolus dose based on TOF monitoring and were paralyzed for a mean duration of 2.6 days with doxacurium or 2.2 days with pancuronium. There was a clinically significant increase in heart rate after the initial bolus dose of pancuronium compared with baseline (120 ± 23 versus 109 ± 22 beats/min, respectively) without any differences after the initial dose of doxacurium (107 ± 21 versus 109 ± 21 beats/min, respectively). Once the drugs were discontinued, the pancuronium group had a more prolonged and variable recovery time (279 ± 229 min) than the doxacurium group (135 ± 46 min).
Mivacurium. Mivacurium is one of the shortest-acting NMBAs currently available. It consists of multiple stereo-isomers and has a half-life of approximately two minutes, allowing for rapid reversal of the blockade. There are little data to support its use as a continuous infusion in the ICU.
Indications
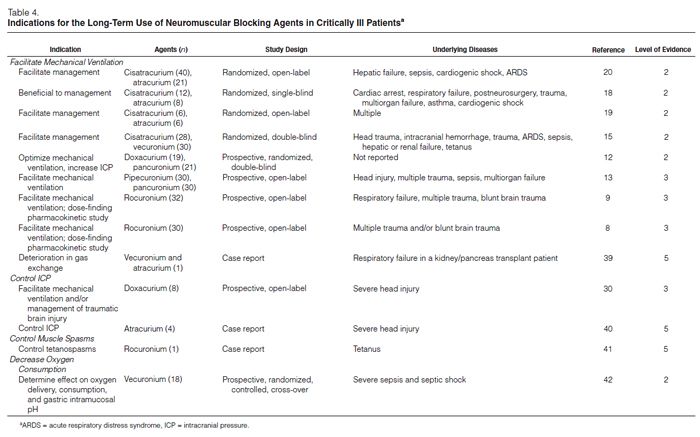
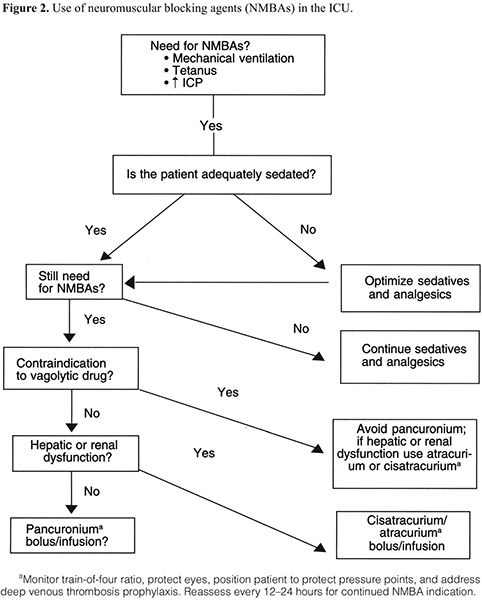
NMBAs are indicated in a variety of situations (Table 4).8,9,12–15,17–21,30,39,42 There have been no studies randomizing patients who are considered candidates for NMBAs to a placebo versus an NMBA. We therefore reviewed many studies comparing one NMBA to another to assess the clinical indications for enrolling patients in these studies. The most common indications for long-term administration of NMBAs included facilitation of mechanical ventilation, control of ICP, ablation of muscle spasms associated with tetanus, and decreasing oxygen consumption (Figure 2). NMBAs are often used to facilitate ventilation and ablate muscular activity in patients with elevated ICP or seizures but have no direct effect on either condition. Patients who are being treated for seizures who also take NMBAs should have electroenceph-alographic monitoring to ensure that they are not actively seizing while paralyzed.
With the exception of atracurium and cisatracurium, which need to be given continuously because of their short half-lives, bolus administration of NMBAs offers potential advantages for controlling tachyphylaxis; monitoring for accumulation, analgesia, and amnesia; and limiting complications related to prolonged or excessive blockade; and improving economics. However, in many ICUs, NMBAs are administered continuously, achieving adequate paralysis and faster recovery with TOF monitoring.
Facilitate Mechanical Ventilation. Numerous reports have described the use of NMBAs to facilitate mechanical ventilation. Most of the reports are limited to case studies, small prospective open-label trials, and small randomized open-label and double-blind trials enrolling a wide variety of critically ill patients to whom NMBAs were given to prevent respiratory dysynchrony, stop spontaneous respiratory efforts and muscle movement, improve gas exchange, and facilitate inverse ratio ventilation. However, none of these reports compared NMBAs to placebos.
Manage Increased ICP. The data supporting the use of NMBAs to control ICP are limited to a case report and an open-label trial. Prielipp30 evaluated doxacurium use in eight patients with severe head injury in an open-label prospective study. NMBAs were given to facilitate ventilation or to manage brain injuries. Patients received an initial bolus injection of doxacurium 0.05 mg/kg followed by a continuous infusion of 0.25 μg/kg/min adjusted to maintain one twitch of the TOF. Doxacurium had no effect on ICP, heart rate, or blood pressure. Infusion rates were similar at the beginning (1 ± 0.1 mg/hr) and at the end (1.3 ± 0.2 mg/hr) of the study. TOF responses returned at 118 minutes; a TOF ratio of 0.7 was measured at 259 ± 24 minutes. No adverse events were reported.
McClelland, et al.40 treated three patients with atracurium for four to six days to manage increased ICP. patients could undergo a neurologic examination within minutes after discontinuing atracurium. No adverse events were reported. There have been no controlled studies evaluating the role of NMBAs in the routine management of increased ICP.
Treat Muscle Spasms. Case studies describe the use of NMBAs in the treatment of muscle contractures associated with tetanus, drug overdoses, and seizures; many were published before 1994.
Anandaciva and Koay41 administered a continuous rocuronium infusion to control muscle tone in patients with tetanus. Muscle spasms recurred at an infusion rate of 8 μg/kg/min, and neither administering a bolus dose of 0.9 mg/kg nor increasing the infusion rate to 10 μg/kg/min controlled the muscle contractures but did increase heart rate. Switching to a different NMBA could control the spasms.
Decrease Oxygen Consumption. Freebairn, et al.42 evaluated the effects of vecuronium on oxygen delivery, oxygen consumption, oxygen extraction ratios, and gastric intramucosal pH in a randomized, placebo-controlled crossover trial in 18 critically ill patients with severe sepsis. Although the infusion of vecuronium achieved an adequate level of paralysis and improved respiratory compliance, it did not alter intramucosal pH, oxygen consumption, oxygen delivery, or oxygen extraction ratios.
Recommended Indications
There are no prospective, randomized, controlled trials assigning patients to an NMBA versus a placebo with a goal of documenting if such patients could be managed by means other than NMBA therapy.
Recommendation: NMBAs should be used for an adult patient in an ICU to manage ventilation, manage increased ICP, treat muscle spasms, and decrease oxygen consumption only when all other means have been tried without success. (Grade of recommendation = C)
Recommended Drugs
There has, in essence, been no study since the last guidelines were published that questions the use of pancuronium for the majority of patients in an ICU. Those prospective, randomized, controlled trials (PRCTs) that have been conducted do not clearly show the benefits of using newer agents or any other agents instead of pancuronium.
There are no well-designed studies with sufficient power to make this a level A recommendation, but there is evidence in the literature that patients on pancuronium fare as well as or better than patients receiving any other NMBA.
The two adverse effects of pancuronium that are commented on frequently are vagolysis and an increase in heart rate. Therefore, patients who would not tolerate an increase in heart rate, i.e., those with cardiovascular disease, should probably receive an NMBA other than pancuronium. The indications for the use of an NMBA must outweigh the risk of tachycardia, and that is based on interpretation of the severity of the patient’s underlying cardiovascular disease. For example, a patient with a history of atrial fibrillation now in sinus rhythm and otherwise hemodynamically stable might better tolerate pancuronium than a patient who is hospitalized with cardiogenic pulmonary edema and managed with mechanical ventilation. The clinician should choose an NMBA on the basis of other patient characteristics. Any benzylisoquinolinium compound or aminosteroidal compound could be substituted for pancuronium in these circumstances.
There are no ideal PRCTs that support this recommendation, but there are data suggesting that patients recover more quickly following administration of cisatracurium or atracurium compared with patients receiving other NMBAs if they have evidence of hepatic or renal disease.
Recommendations: The majority of patients in an ICU who are pre-scribed an NMBA can be managed effectively with pancuronium. (Grade of recommendation = B)
For patients for whom vagolysis is contraindicated (e.g., those with cardiovascular disease), NMBAs other than pancuronium may be used. (Grade of recommendation = C)
Because of their unique metabolism, cisatracurium or atracurium is recommended for patients with significant hepatic or renal disease. (Grade of recommendation = B)
Monitoring
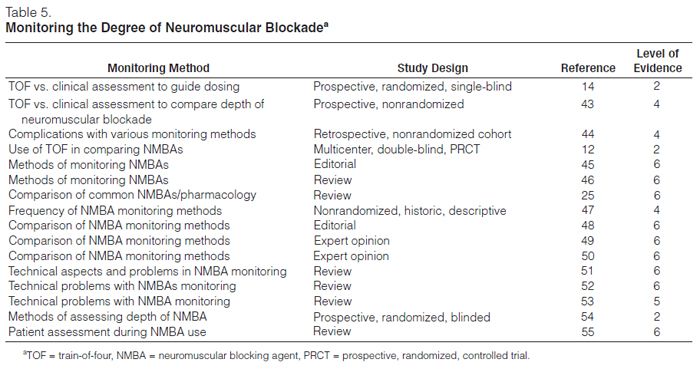
Monitoring neuromuscular blockade is recommended (Table 5).12,14,43–55 Monitoring the depth of neuromuscular blockade may allow use of the lowest NMBA dose and may minimize adverse events. No PRCT has reported that reducing the dose of an NMBA can prevent persistent weakness. Despite this lack of evidence and the lack of a standardized method of monitoring, assessment of the depth of neuromuscular blockade in ICU patients is recommended.43
Visual, tactile, or electronic assessment of the patient’s muscle tone or some combination of these three is commonly used to monitor the depth of neuromuscular blockade. Observation of skeletal muscle movement and respiratory effort forms the foundation of clinical assessment; electronic methods include the use of ventilator software allowing plethysmographic recording of pulmonary function to detect spontaneous ventilatory efforts and “twitch monitoring,” i.e., the assessment of the muscular response by visual, tactile, or electronic means to a transcutaneous delivery of electric current meant to induce peripheral nerve stimulation (PNS).
Since the last practice guidelines were published, only two studies have examined the best method of monitoring the depth of neuromuscular blockade, and none have compared the efficacy or accuracy of specific techniques. The first study was a prospective, randomized, single-blinded trial of 77 patients in a medical ICU who were administered vecuronium based on either clinical parameters (patient breathed above the preset ventilatory rate) or TOF monitoring, with a goal of one of four twitches.44 PNS resulted in a significantly lower total dose and lower mean infusion rate of NMBA as well as a faster time to recovery of neuromuscular function and spontaneous ventilation.
A second study sought to compare the depth of blockade induced by atracurium either by “best clinical assessment” (i.e., maintenance of patient-ventilator synchrony and prevention of patient movement) or TOF monitoring (with a goal of three of four twitches). Analysis of the 36 medical ICU patients in this prospective, nonrandomized trial revealed no difference in the total dose, mean dose, or the mean time to clinical recovery.43 This may have been due to sample size or study design.
An additional study examining the results of the implementation of a protocol using PNS to monitor the level of blockade in patients receiving a variety of NMBAs found a reduction in the incidence of persistent neuromuscular weakness.49
Other methods of electronic monitoring of the depth of blockade are fraught with difficulties; TOF monitoring of PNS remains the easiest and most reliable method available,43,44,46–50 despite its shortcomings and technical pitfalls.51–53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree