Powders, granules and granulation
Michael E. Aulton and Malcolm P. Summers
Chapter contents
Powdered and granulated products as dosage forms
Powders and granules for oral administration
Powders for other routes of administration
Preparations requiring further treatment at time of dispensing
Granules used as an intermediate in tablet manufacture
Pharmaceutical technology of granule production
Pharmaceutical granulation processes
Mechanisms of granule formation
Pharmaceutical granulation equipment and processes
Key points
• Powders and granules are themselves dosage forms.
• Powders are also used for inhalation (pulmonary or nasal) and for external use (dusting powders).
Introduction
The scientific aspects of powders and powder technology have been discussed in Part 2 of this book. This chapter discusses the application of powders and granulated products in pharmaceutical dosage forms. Powders and granules are used as dosage forms in their own right, but by far the greatest use of granules in pharmaceutical manufacturing is as an intermediate during the manufacture of compressed tablets.
The term ‘powder’, when used in the context of a dosage form, describes a formulation in which a drug powder has normally been mixed with other powdered excipients to produce a final product. The function of the added excipients depends upon the intended use of the product. Colouring, flavouring and sweetening agents, for example, may be added to powders for oral use.
Granules that are used as a dosage form comprise powder particles that have been aggregated to form larger particles sufficiently robust to withstand handling.
Granulation is the process in which dry primary powder particles (i.e. single, discrete powder particles) are processed to adhere to form larger multi-particle entities called granules. Pharmaceutical granules typically have a size range between 0.2 and 4.0 mm, depending on the subsequent use of the granules. In the majority of cases, when granules will be made as an intermediate product, they have a size range towards the lower end of this spectrum – typical between 0.2 and 0.5 mm. When prepared for use as a dosage form in their own right, they are usually much larger (typically 1–4 mm).
After granulation, the granules will either be packaged (when used as a dosage form) or they may be mixed with other excipients prior to tablet compaction or capsule filling.
Reasons for granulation
The reasons why granulation is often necessary are as follows:
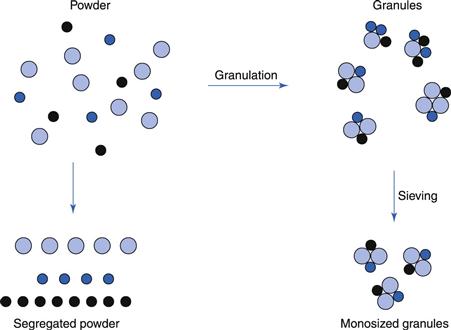
To prevent segregation of the constituents of the powder mix
Segregation (or demixing, discussed in Chapter 11) occurs primarily due to differences in the size and/or density of the components of the mix; the smaller particles and/or denser particles concentrate at the base of a container with the large particles and/or less dense above them. An ideal granulation will contain all the constituents of the mix in the correct proportion in each granule, and if this is achieved segregation of individual ingredients will not occur (Fig. 28.1).
It is also important to control the particle size distribution of the granules because, although the individual components may not segregate, if there is a wide size distribution of the granules themselves, the granules may segregate. If this occurs in the hoppers of sachet-filling machines, capsule-filling machines or tabletting machines, products having large weight variations will result. This is because these machines fill by volume rather than weight. If different regions in the hopper contain granules of different sizes (and hence different bulk density), a given volume from each region will contain a different weight of granules. This will lead to an unacceptable variability of the drug content within the batch of finished product even if the drug is evenly distributed, weight per weight, through individual granules.
To improve the flow properties of the mix
Many powders, because of their small size, irregular shape or surface characteristics, are cohesive and do not flow well. Poor powder flow will also often result in a wide weight variation within the final product due to variable fill of tablet dies, etc. The resulting granules produced from irregular particles will be larger and more isodiametric, both factors contributing to improved flow properties (discussed more fully in Chapter 12).
To improve the compaction characteristics of the mix
Some primary powder particles are difficult to compact into tablets even if a readily compactable adhesive is included in the blend. Granules of the same formulation are often more easily compacted and produce stronger tablets. This is associated with the method employed to produce the granule and its resulting structure. Often solute migration (see Chapter 29) may occur during the post-wet granulation drying stage and this can result in a binder-rich outer layer to the granules. This in turn leads to direct binder–binder bonding which assists the consolidation of weakly bonding materials.
Other reasons
The above are the primary reasons for the granulation of pharmaceutical products but there are others which may necessitate the granulation of powdered material:
Powdered and granulated products as dosage forms
Powdered and granulated products are dispensed in many forms and these are discussed below. The advantages of this type of preparation as a dosage form are as follows:
The disadvantages of powdered and granulated dosage forms are as follows:
1. Bulk powders or granules (i.e. where doses are not pre-divided into individual aliquots) are far less convenient for the patient to carry than a small container of tablets or capsules, and are as inconvenient to self-administer as liquid preparations, such as mixtures. Modern packaging methods for divided preparations, such as heat-sealable laminated sachets (Chapter 47), mean that individual doses can be carried conveniently.
Powders and granules for oral administration
Oral powders
Oral powders are preparations consisting of solid, loose, dry particles of varying degrees of fine particle size. They contain one or more active substances, with or without excipients and, if necessary, approved colouring matter and flavouring. They are generally administered in or with water or another suitable liquid, or they may also be swallowed directly. They are presented as single-dose or multidose preparations in suitable containers.
Multidose oral powders are packed into a suitable bulk container, such as a wide-mouthed glass jar. They require the provision of a measuring device capable of delivering the quantity prescribed. Because of the difficulty in precisely measuring single doses from this type of preparation the constituents are usually relatively non-toxic medicaments with a large dose. Relatively few proprietary examples exist, although many dietary/food supplements are packed in this way.
Each dose of a single-dose powder is enclosed in an individual container, for example a sachet or a vial. Traditionally, single doses were wrapped in paper. This was unsatisfactory for most products, particularly if the ingredients were hygroscopic, volatile or deliquescent. Modern packaging materials of foil and plastic laminates have replaced such paper wrappings; they offer superior protective qualities and are amenable to use on high-speed packing machines. However, some paper-wrapped powders continue in over-the-counter products.
In the manufacture of oral powders, effort is made to ensure a suitable particle size is used with regard to the intended use. Additionally during manufacture, packaging, storage and distribution of oral powders, suitable means must be taken to ensure microbial quality. All powders and granules should be stored in a dry place to prevent deterioration due to ingress of moisture. Even if hydrolytic decomposition of ingredients does not occur, the particles will adhere and cake, producing an inelegant, often unusable product.
Effervescent powders
Effervescent powders are presented as single-dose or multidose preparations and generally, in addition to the drug, contain acid substances and carbonates or hydrogen carbonates which react rapidly and effervesce when the patient adds the powder to water to produce a draught. Citric acid plus sodium bicarbonate is a common combination that releases carbon dioxide. The drug is quickly dissolved or dispersed in the water before administration.
It is preferred that effervescent powders are packed in individual dose units in airtight containers (laminated sachets are ideal, Chapter 47). It is important to protect the powder from the ingress of moisture during manufacture and on subsequent storage to prevent the reaction occurring prematurely.
Granules
One disadvantage of powders is that, because of particle size differences, the ingredients may segregate (see Chapter 11), either in the hoppers of packaging machines or on storage in the final bulk container. If this happens, the product will be non-uniform and the patient will not receive the same dose of the ingredients on each occasion. This can be minimized by efficient granulation of the mixed powders.
Granules are preparations consisting of solid, dry aggregates of powder particles sufficiently resistant to withstand handling. They are intended for oral administration. Some are swallowed as such, some are chewed and some are dissolved or dispersed in water or another suitable liquid before being administered.
Granules contain one or more active substances with or without excipients and, if necessary, suitable colouring and flavouring substances. They are mainly used for low-toxicity, high-dose drugs. Methylcellulose Granules, for example, are used as a bulk-forming laxative and have a dose of 1–4 g daily. Many proprietary preparations contain similar bulk-forming laxatives.
Granules are presented as single-dose or multidose preparations. Each dose of a multidose preparation is administered by means of a device suitable for measuring the quantity prescribed. For single-dose granules, each dose is enclosed in an individual container, for example a sachet or a vial. If the preparation contains volatile ingredients or the contents have to be protected, they should be stored in an airtight container. For example, Methylcellulose Granules should be kept in a wide-mouthed, airtight container.
There are several categories of granules:
Effervescent granules.
Effervescent granules are uncoated granules generally containing acid substances and carbonates or hydrogen carbonates which react rapidly in the presence of water to release carbon dioxide. They are intended to be dissolved or dispersed in water before administration. The effervescence and subsequent disintegration of the granules should be complete within 5 minutes at which time the granule ingredients should be either dissolved or dispersed in the water. Effervescent granules should be stored in an airtight container.
Coated granules.
Coated granules consist of granules coated with one or more layers of mixtures of various excipients. The substances used as coatings (generally polymers) are usually applied as a solution or suspension in conditions in which evaporation of the vehicle occurs leaving a film of coating (see Chapter 32). A suitable test should be carried out to demonstrate the appropriate release of the active substance(s), for example one of the tests described in Chapter 35.
Modified-release granules.
Modified-release granules are coated or uncoated granules that contain special excipients or which are prepared by special procedures, or both, designed to modify the rate, the place or the time at which the active substance or substances are released.
Modified-release granules may have prolonged-release or delayed-release properties. A suitable test must be carried out to demonstrate the appropriate kinetics and extent of the release of the active substance(s).
Gastro-resistant granules.
Gastro-resistant granules (also referred to as enteric-coated granules) are delayed-release granules that are intended to resist the gastric fluid and to release the active substance(s) in the intestine fluid. This is generally achieved by covering the granules with a gastro-resistant polymer (see Chapters 31 and 32). Again a suitable test should be carried out to demonstrate the appropriate release of the active substance(s).
Powders for other routes of administration
Powders for inhalation
The use of dry-powder systems for pulmonary drug delivery is now extensive. This dosage form has developed into one of the most effective methods of delivering active ingredients to the lung for the treatment of asthma and chronic obstructive pulmonary disease. Its popularity is reflected in the number of commercial preparations available in a number of sophisticated and increasingly precise delivery devices. Pulmonary delivery is discussed fully in Chapter 37.
Nasal powders
Nasal powders are medicated powders intended for inhalation into the nasal cavity by means of a suitable device. Some potent drugs are presented in this way because they are rapidly absorbed when administered as a fine powder via the nose (see Chapter 38 for a detailed discussion of the nasal route of administration). To enhance convenience and ensure that a uniform dose is delivered on each occasion, delivery devices have been developed. Sufficient drug for one dose may be presented in a hard gelatin capsule diluted with an inert, soluble diluent such as lactose. The capsule is placed in the body of the nasal delivery device and is broken when the device is assembled. The drug is inhaled, via the nose, by the patient as a fine powder. The size of the particles is such as to localize their deposition in the nasal cavity and is verified by adequate methods of particle-size determination.
Powders for external use
Powders for cutaneous application (topical powders)
Powders for cutaneous application are preparations consisting of solid, loose, dry particles of varying degrees of fineness. They contain one or more active substances, with or without excipients and, if necessary, appropriate colouring matter.
Powders for cutaneous application are presented as single-dose powders or multidose powders. They should be free from grittiness. Powders specifically intended for use on large open wounds or on severely injured skin must be sterile.
Multidose powders for cutaneous application may be dispensed in sifter-top containers, containers equipped with a mechanical spraying device or in pressurized containers.
In the manufacture of powders for cutaneous application, measures should be taken to ensure a suitable particle size (determined and controlled by sieving) with regard to the intended use. Additionally, suitable measures should be taken to ensure their microbial quality and if the label indicates that the preparation is sterile, it must comply with a test for sterility.
Sterile powders used in cutaneous application must be prepared using materials and methods designed to ensure sterility and to avoid the introduction of contaminants and the growth of micro-organisms.
Dusting powders
Dusting powders contain ingredients used for therapeutic, prophylactic or lubricant purposes and are intended for external use. Only sterile dusting powders should be applied to open wounds.
Dusting powders for lubricant purposes or superficial skin conditions need not be sterile but they should be free from pathogenic organisms. As minerals such as talc and kaolin may be contaminated at source with spores of organisms causing tetanus and gangrene, these should be sterilized before they are incorporated into the product. Talc Dusting Powder is a sterile cutaneous powder containing starch and purified talc in which the talc is sterilized before incorporation with the starch, or the final product is subject to a suitable terminal sterilization procedure.
Dusting powders are normally dispensed in glass or metal containers with a perforated lid. The powder must flow well from such a container, so that it can be dusted over the affected area. The active ingredients must therefore be diluted with materials having reasonably good flow properties, e.g. purified talc or maize starch.
Hexachlorophene Dusting Powder contains an antibacterial agent and Talc Dusting Powder is used as a lubricant to prevent chafing. Proprietary products are available, usually for the treatment of bacterial or fungal infections, e.g. Canesten® Powder (clotrimazole) is used as an antifungal agent.
Dusting powders are powders for cutaneous application which have a suitable fineness. An example is Talc Dusting Powder, which is a mix of 10% of starch and 90% of Purified Talc, where the particle size is controlled by size separation using, typically, a 250 µm sieve.
Ear powders
Powders containing active ingredients can also be administered to the ear. Ear powders normally have to comply with the pharmaceutical requirements for powders for cutaneous application. They are supplied in containers fitted with a suitable device for application.
Preparations requiring further treatment at time of dispensing
Some preparations for oral use are prepared from powders or granules to yield oral solutions or suspensions using a suitable vehicle. This may be performed at the dispensing stage or by the patient prior to administration. The vehicle for any preparations for oral use is chosen having regard to the nature of the active substance(s) and to provide organoleptic characteristics appropriate to the intended use of the preparation.
Several categories of preparations may be distinguished:
Powders and granules for solution or suspension
Powders and granules for the preparation of oral solutions or suspensions generally conform to the definitions in the normal pharmacopoeial standards for oral powders or granules as appropriate. They may contain excipients, in particular to facilitate dispersion or dissolution and to prevent caking. After dissolution or suspension, the resulting product should comply with the requirements for oral solutions or oral suspensions, as appropriate.
The label should explain the method of preparation of the solution or suspension from the powder or granules, and the conditions and the duration of storage after reconstitution.
Powders and granules for syrups
Syrups are aqueous preparations characterized by a sweet taste and a viscous consistency. They may contain sucrose at a concentration of at least 45%. The sweet taste can also be obtained by using other polyols or sweetening agents. Syrups usually contain aromatic or other flavouring agents.
All of the necessary ingredients for the syrup may be manufactured and stored in the dry powdered or granular state and then reconstituted (usually by the addition of water alone) at the time of dispensing or administration. After dissolution, the resulting syrup must comply with the normal pharmacopoeial requirements for syrups.
Antibiotic syrups.
For patients who have difficulty taking capsules and tablets, e.g. young children, a liquid preparation of a drug offers a suitable alternative. Unfortunately, many antibiotics are physically or chemically unstable when formulated as a solution or suspension. The method used to overcome this instability problem is to manufacture the dry ingredients of the intended liquid preparation in a suitable container in the form of a powder or granules. When the product is dispensed, a given quantity of water is added to reconstitute the solution or suspension. This enables sufficient time for warehousing and distribution of the product and storage at the pharmacy without degradation. Once it is reconstituted, the patient must be warned of the short shelf-life. A shelf-life of 1–2 weeks for the reconstituted antibiotic syrup should not be a serious problem for the patient as the dosing would normally be complete by then. Examples are Amoxicillin Oral Suspension and Erythromycin Ethylsuccinate Oral Suspension.
Powders for oral drops
Oral drops are solutions, emulsions or suspensions that are administered in small volumes, such as in drops, by the means of a suitable device. Powders for the preparation of oral drops would have to conform to requirements of all other oral powders. They may contain excipients to facilitate dissolution or suspension in the prescribed liquid, or to prevent caking.
After dissolution or suspension, they comply with the specific pharmacopoeial requirements for pre-prepared oral drops. If the dose is measured in drops, the label should also state the number of drops per millilitre or per gram of preparation.
Powders for injection
Injections of medicaments that are unstable in aqueous solution must be made immediately prior to use. The ingredients are presented as sterile powders in ampoules or vials. Sufficient diluent, e.g. sterile Water for Injections, is added from a second container to produce the required drug concentration and the injection is used immediately. The powder may contain suitable excipients in addition to the drug, e.g. sufficient additive to produce an isotonic solution when the injection is reconstituted.
Powders for injection are most often manufactured by a freeze drying process (Chapter 29). The sterilization of these ‘lyophilized powders’ is described in Chapter 17 and their use as parenteral products is discussed in more detail in Chapter 36.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree