Postgastrectomy and Postvagotomy Syndromes
David W. Mercer
Matthew R. Goede
Introduction
Although gastric surgery has decreased over the past several decades given the improved understanding of the pathophysiology and treatment of peptic ulcer disease, there are still a significant number of patients who have had gastric surgery for neoplasm, trauma, or morbid obesity and are at the risk of developing these syndromes.
The development of these post-operative syndromes can be attributed to the anatomical changes that occur following surgery. They are due to either loss of the gastric reservoir, loss of pyloric function, transection of the vagus nerves, or are sequelae of restoring gastrointestinal continuity.
The development of post-operative symptoms is fairly frequent following gastric surgery. About 25% of the patients report some kinds of symptoms, with about 2% to 5% of the patients having symptoms severe enough to be disabling. As experience with postgastrectomy and postvagotomy syndromes decreases, we have to examine the experience of past surgeons and learn lessons from the development of their techniques. Fortunately, we have the benefit of advances in pharmacology, endoscopy, and imaging to enhance our diagnostic and treatment plans.
Gastric Dysfunction
Early Dumping Syndrome
Early dumping syndrome is classified as early secondary to its proximity to the ingestion of a meal, usually occurring within 10 to 30 minutes of a meal. Gastrointestinal symptoms include epigastric bloating, nausea, vomiting, crampy abdominal pain, and explosive diarrhea. Cardiovascular symptoms are also present and include palpitations, tachycardia, diaphoresis, dizziness, flushing, hypotension, syncope, and blurred vision. Generally, the constellation of symptoms in early dumping syndrome is all that is necessary to make the diagnosis in the patients with a history of a gastrectomy. Occasionally, additional testing is required, especially if one is entertaining surgical intervention to improve early dumping syndrome. Either a contrast upper gastrointestinal study or a nuclear medicine gastric emptying study could be used to demonstrate rapid gastric emptying. Alternatively, a monitored oral glucose challenge (200 mL of 50% glucose) can be done which elicits the constellation of symptoms in the patients with early dumping.
Early dumping syndrome can be seen to some degree in about 25% of the patients who have had gastric surgery. Early dumping syndrome is more common following gastrectomy that removes more than two-thirds of the stomach. It is more commonly seen following Billroth II reconstruction than Bilroth I reconstruction, but can occur in both. In the patients who have had the combination of a greater than two-thirds gastrectomy and a Billroth II reconstruction, 50% to 60% will have some degree of dumping syndrome. In addition, early dumping syndrome can occur following vagotomy and drainage procedures, especially if the drainage procedure is a large gastroenterostomy or a Finney pyloroplasty. Early dumping syndrome can also be seen in the patients who have undergone Roux-en-Y gastric bypass for weight loss surgery.
Early dumping syndrome is believed to be due to the rapid delivery of high osmolarity chyme into the proximal small intestine due to loss of the pylorus and loss of vagally mediated gastric receptive relaxation leading to less chemical and mechanical breakdown of the food bolus. This in turn leads to an extracellular fluid shift into the intestinal lumen in order to achieve isotonicity. The subsequent luminal distension initiates autonomic responses which are triggered by the decrease in circulating plasma volume. However, not all studies have demonstrated a clear correlation between reduced plasma volume and severity of the symptoms. For example, some studies have demonstrated that humoral factors, such as serotonin from the small intestine, may be released in the patients experiencing dumping syndrome that are not released in asymptomatic patients who have had a gastrectomy. Other studies have shown that serotonin antagonists improve dumping syndrome symptoms. Bradykinin has also been implicated in early dumping syndrome as exogenous bradykinin induces many of the same vasomotor symptoms seen in the syndrome. Enteroglucagon levels have also been shown to be more elevated in response to an enteral glucose load in the patients with early dumping syndrome when compared to patients without the syndrome. Enteroglucagon inhibits sodium and water absorption from the intestinal lumen, which could contribute to the diarrhea seen with early dumping syndrome. Neurotensin, vasoactive intestinal peptide, cholecystokinin, peptide YY, and atrial natriuretic peptide have also been evaluated for their participation in early dumping syndrome.
Most patients with early dumping syndrome can be managed by dietary modifications. Patients should be told to avoid consuming meals with large amounts of sugars and increase their protein consumption. Patients should also make behavioral changes to prolong gastric emptying, for example, not drinking liquids during the same time as consumption of solid food. Positional changes like reclining or lying down following meals can also retard gastric emptying by eliminating the effect of gravity on the stomach. Frequently, the symptoms of early dumping syndrome will decrease over time.
Pharmacologic therapies are usually reserved for the patients with debilitating early dumping syndrome. Serotonin antagonists like cyproheptadine and methysergide have shown some effectiveness when taken before meals. However, the side effects of these drugs limit their use, given the escalating doses required to maintain efficacy. The somatostatin analog, octreotide, has been shown to inhibit the hormonal cascade associated with early dumping syndrome and is quite effective in minimizing the vasomotor symptoms of early dumping syndrome. Octreotide also slows both gastric emptying and intestinal transit times, thereby controlling the associated diarrhea.
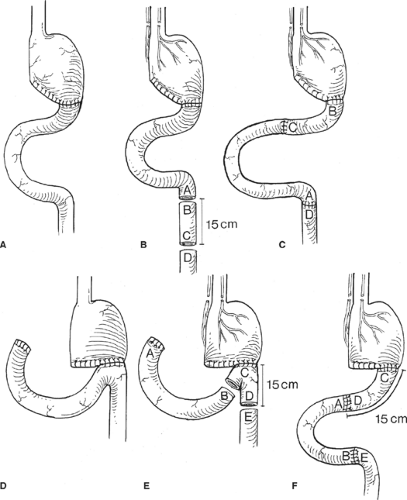
It is exceedingly rare for the patients to continue to experience debilitating early dumping syndrome following dietary modifications, behavioral changes, and pharmacologic therapy. However, in 1% to 2% of the patients who do fail non-operative management, surgical intervention may relieve some of the symptoms. One procedure is to interpose a 10 to 20 cm isoperistaltic segment of jejunum between the remaining stomach and the small bowel (Fig. 1) in an attempt to increase the size of the gastric reservoir. This segment of jejunum will dilate, thereby increasing the gastric reservoir and function to delay gastric emptying. A second procedure designed to slow gastric emptying and create a “neo-pylorus” involves placing a 10 cm
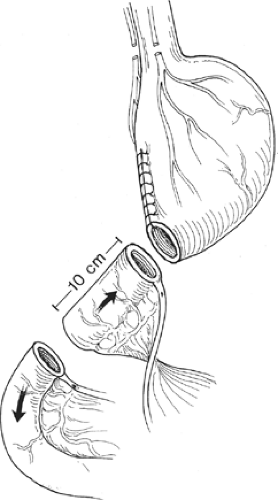
antiperistaltic segment of jejunum between the remaining stomach and the previous gastroenteric anastomosis (Fig. 2). This segment of jejunum will act as a “brake” to gastric emptying and allow more time for gastric secretions and gastric peristalsis to break down the food bolus. Antiperistaltic segments should be kept <10 cm in length, as longer segments can develop their own complications such as a functional gastric outlet obstruction. A third procedure would be to convert the patient to a long limb Roux-en-Y reconstruction which has demonstrated success in about 75% of the patients in alleviating their symptoms.
antiperistaltic segment of jejunum between the remaining stomach and the previous gastroenteric anastomosis (Fig. 2). This segment of jejunum will act as a “brake” to gastric emptying and allow more time for gastric secretions and gastric peristalsis to break down the food bolus. Antiperistaltic segments should be kept <10 cm in length, as longer segments can develop their own complications such as a functional gastric outlet obstruction. A third procedure would be to convert the patient to a long limb Roux-en-Y reconstruction which has demonstrated success in about 75% of the patients in alleviating their symptoms.
Late Dumping Syndrome
As its name suggests, late dumping syndrome occurs 2 to 3 hours after a meal. Late dumping syndrome is much less common than early dumping syndrome, but like early dumping syndrome, late dumping syndrome is due to rapid gastric emptying. When large amounts of sugar or carbohydrates are quickly delivered to the small intestine, they are rapidly absorbed. This rapid absorption leads to hyperglycemia and an increase in enteroglucagon release from small bowel mucosal cells, which causes a large release in insulin. However, this exaggerated release of insulin results in rebound hypoglycemia which in turn elicits the release of catecholamines, causing tachycardia, diaphoresis, tremulousness, dizziness, and confusion—the classic symptoms associated with hypoglycemia.
Late dumping syndrome is also mainly controlled through dietary modifications and usually requires that patients avoid sugars and large carbohydrate loads. Patients who remain symptomatic can be treated with α-glucosidase inhibitors which prevent sugar and starch breakdown, thereby inhibiting absorption. In desperate cases, an antiperistaltic jejunal limb or Roux-en-Y can be performed to slow gastric emptying.
Small Stomach Syndrome
Small stomach syndrome is due to the decreased reservoir present after a large partial gastrectomy. Patients experience pain, either abdominal or chest, following ingestion of a meal and may also suffer from nausea, vomiting, and weight loss. Symptoms are likely due to the decreased distensibility of the proximal stomach. In a patient with a
subtotal gastrectomy, the vagal nerves are transected higher on the stomach which prevents receptive relaxation of the stomach and limits the ability of the proximal stomach to distend. This lack of distension frequently becomes apparent as the patient begins to increase their oral intake following surgery.
subtotal gastrectomy, the vagal nerves are transected higher on the stomach which prevents receptive relaxation of the stomach and limits the ability of the proximal stomach to distend. This lack of distension frequently becomes apparent as the patient begins to increase their oral intake following surgery.
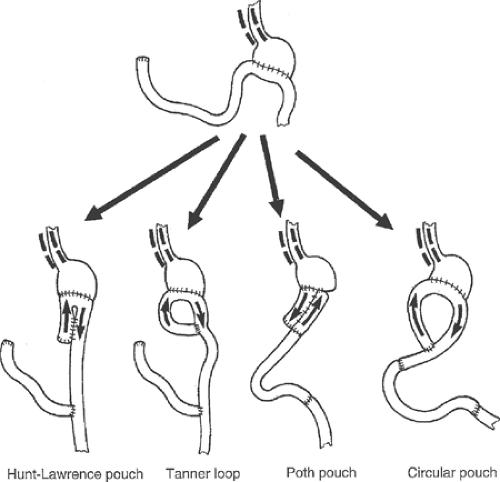
Small stomach syndrome is usually treated through small frequent feedings. If patients continue to be intolerant of meals, liquid enteral nutrition can be provided orally, or if need be, through a distal feeding tube. Over time, proximal gastric pouches may dilate some, thereby improving symptoms in those patients. In the patients who have a syndrome that is refractory to dietary changes and in the patients suffering from chronic malnutrition, surgery can be considered to increase the reservoir size. As in early dumping syndrome, an isoperistaltic jejunal interposition can be used to increase the reservoir. Also, several versions of jejunal J-pouch (Hunt-Lawrence, or Poth pouches, as shown in Fig. 3) can be attempted to increase the capacity of the stomach.
Metabolic Disturbances
Anemia is the most common metabolic disturbance following gastrectomy. Iron deficiency anemia is the most common cause after gastrectomy, followed by vitamin B-12 deficiency. The absorption of iron occurs primarily in the duodenum. Consequently, any procedure that bypasses the duodenum (Roux-en-Y, Billroth II) results in impaired absorption of iron. In addition, because iron absorption requires acidic conditions, any gastric procedure inhibiting acid secretion (vagotomy, antrectomy) could lead to iron deficiency anemia. Iron deficiency anemia following a gastrectomy is managed by supplementation of iron orally, along with vitamin C. Vitamin C reduces ferric to ferrous iron, which helps with the uptake of iron into the mucosal cells. Vitamin C also has been shown to maintain iron in a more soluble form, thereby leading to improved absorption throughout the GI tract.
A deficiency in vitamin B-12 can lead to megaloblastic anemia. This occurs due to the loss of intrinsic factor which is needed to bind and absorb vitamin B-12 in the ileum. In order to become deficient in intrinsic factor, >50% of the stomach needs to be resected which results in loss of parietal cells—the cells responsible for intrinsic factor production. Vitamin B-12 deficiency is treated by parenteral administration, usually by intramuscular injection.
Weight loss is also frequently seen following gastrectomy. However, rarely does significant malnutrition develop. The risk for significant malnutrition increases with the extent of the gastrectomy. The cause for this is likely twofold. The larger the partial gastrectomy performed, the smaller the reservoir present, restricting the amount of food intake at a given time. Also, the larger the partial gastrectomy performed the higher the vagus nerves are divided, which likely effects hormone secretion, gastric function, and the perception of hunger. Conservative treatment entails providing the patient with caloric and nutrient-dense food or supplements. If the patient’s weight and protein stores do not improve, more aggressive measures (enteral tube feeding) may become necessary.
Dysfunction Due to Gastrointestinal Reconstruction
Afferent Loop Syndrome
Afferent loop syndrome is due to partial obstruction of the afferent loop of a Bilroth II or Roux-en-Y reconstruction. This obstruction prevents the afferent limb, or biliopancreatic limb, from adequately draining. This obstruction can be from adhesions; stenosis or angulation of the afferent limb at the anastomosis; edema immediately post-operatively at the anastomosis; volvulus; or the afferent limb becoming incarcerated in an internal hernia (Fig. 4).
Because of differences in anatomy, patients who have undergone Roux-en-Y reconstruction and Bilroth II reconstruction present differently with afferent loop syndrome. In patients with afferent loop syndrome following Roux-en-Y reconstruction, biliopancreatic secretions accumulate in the afferent limb, especially after meals, causing upper abdominal cramping and pain due to obstruction. The symptoms resolve, often quite suddenly, once the pressure in the distended limb overcomes the pressure in the obstructed portion of the limb and luminal fluid decompresses into the small intestine. In the patients with afferent loop syndrome following Bilroth II reconstruction, the same upper abdominal cramping and pain occurs. However, in contrast to the patient with Roux-en-Y, when the afferent limb decompresses, luminal fluid empties into the stomach. The rapid accumulation of fluid into the stomach causes forceful vomiting of bilious material followed by immediate relief of symptoms. The emesis usually lacks the presence of any food because the bolus has already passed down the efferent limb prior to enough pressure building up to decompress the afferent limb. In these patients, the
post-prandial waxing and waning pain is frequently misdiagnosed as symptomatic cholelithiasis or biliary dyskinesia. Acute afferent limb syndrome can also be misdiagnosed as acute pancreatitis. The patient can develop hyperamylasemia and acute abdominal pain from complete obstruction of the afferent limb.
post-prandial waxing and waning pain is frequently misdiagnosed as symptomatic cholelithiasis or biliary dyskinesia. Acute afferent limb syndrome can also be misdiagnosed as acute pancreatitis. The patient can develop hyperamylasemia and acute abdominal pain from complete obstruction of the afferent limb.
If the clinical findings are subtle, further workup can be performed. A CT scan of the abdomen can sometimes demonstrate a larger than expected, fluid-filled afferent limb. On endoscopy, afferent loop syndrome can be suggested by difficulty in intubating the afferent limb, consistent with obstruction. Alternatively, a radionuclide HIDA scan can show excretion by the biliary system but then stagnation upon entering the afferent limb.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree