Post-transplant Lymphoproliferative Disorder, Monomorphic
Pei Lin, MD
L. Jeffrey Medeiros, MD
Key Facts
Terminology
Monomorphic PTLDs fulfill criteria for lymphomas as observed in immunocompetent patients
Etiology/Pathogenesis
Epstein-Barr virus (EBV) infection plays important role in pathogenesis
˜ 80% of all PTLDs are EBV(+); usually type A
Risk factors for developing PTLDs
EBV seronegativity before transplant; young age
Degree of overall immunosuppression
Types of immunosuppression or organs transplanted
Clinical Issues
Lymphadenopathy &/or extranodal sites
EBV(+) PTLDs usually occur < 5 years after transplant
EBV(-) PTLDs develop later (median: 50 months)
3-step approach is taken for treating PTLD patients
Reduction of immunosuppression
Single agent rituximab (anti-CD20 antibody)
Cytotoxic chemotherapy
Microscopic Pathology
˜ 80% monomorphic PTLDs are of B-cell origin
Diffuse large B-cell lymphoma is most common
˜ 15% are NK/T-cell
˜ 5% plasma cell neoplasms and Hodgkin lymphoma
Ancillary Tests
Karyotypic aberrations are detectable in most cases
Monomorphic B-cell PTLD: Monoclonal Ig rearrangements
NK/T-cell PTLDs: Monoclonal TCR rearrangements
TERMINOLOGY
Abbreviations
Post-transplant lymphoproliferative disorder (PTLD)
Synonyms
Post-transplantation lymphoproliferative disease (PTLD)
Definitions
PTLDs are plasmacytic or lymphoid proliferations that occur as a result of immunosuppressive therapy following solid organ or bone marrow transplantation
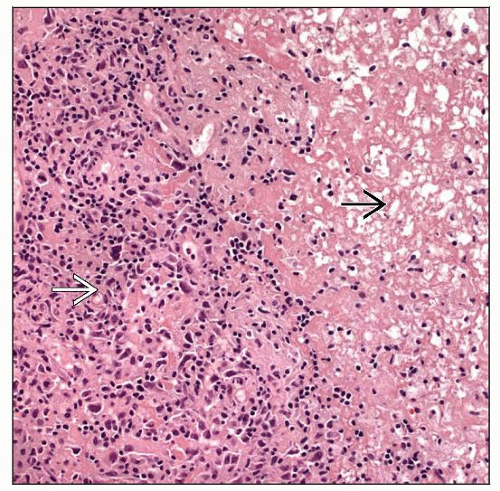
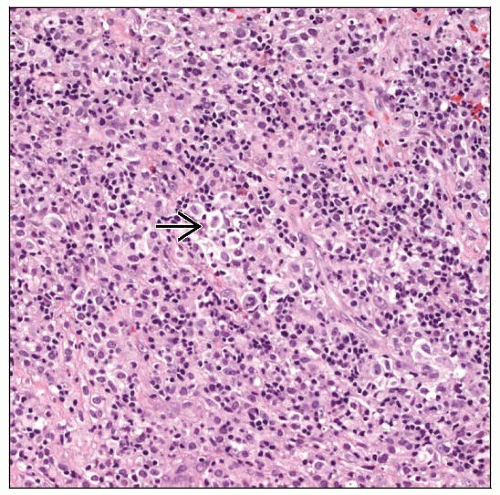
Monomorphic PTLDs fulfill criteria for lymphomas as observed in immunocompetent patients
All cells appear to be transformed but lesions are not completely monotonous
Plasmacytoid/plasmacytic differentiation &/or pleomorphism of neoplastic cells are common
ETIOLOGY/PATHOGENESIS
Infectious Agents
Epstein-Barr virus (EBV) infection plays important role in pathogenesis
˜ 80% of all PTLDs are EBV(+); usually type A
Prior to onset of PTLDs
Serum EBV antibody titers and EBV DNA levels in blood increase
Numbers of EBV(+) cytotoxic T cells decrease prior to onset of PTLD
Therapy with EBV-specific T cells effective in subset of patients
EBV genomes are monoclonal shown by EBV terminal repeat analysis
Virus is present prior to monoclonal expansion
EBV can transform germinal center B cells
Extends lifespan of B cells
Increases likelihood of additional genetic abnormalities that confer growth advantage
EBV latent membrane protein (LMP)1 and LMP2A proteins activate B-cell receptor and intracellular signaling pathways
Decreased Host Immunosurveillance Resulting from Therapeutic Immunosuppression
Underlying immune deficiency can be involved (e.g., cystic fibrosis, hepatitis C infection)
Cumulative amount of immunosuppression
Cyclosporin A, antithymocyte globin (ATG) or OKT3 monoclonal antibodies
Type of organ transplanted
Multiorgan > lung > liver > heart > pancreas > kidney > bone marrow and stem cell transplantation
Kidney transplant patients are more susceptible to NK/T-cell lymphoma and EBV(-)
Stem cell transplant patients are more susceptible to Hodgkin lymphoma
T-cell depletion in donor allograft increases risk
Cell of Origin
B-cell PTLDs arise from germinal center (GC) or post-GC B-cells
Cell of origin of T-cell PTLDs is unknown
90% of PTLD in recipients of solid organ transplants are of host origin
Most PTLD in recipients of bone marrow/stem cell transplant are of donor origin
Risk Factors for PTLDs in General
EBV seronegativity before transplant
Degree of overall immunosuppression
Higher risk if multiple regimens are used or if patients receive multiple transplants
Type of immunosuppression
Type of organs transplanted
Highest risk in patients receiving intestinal or multi-organ transplants
Lowest risk in patients who receive kidney allograft
Likely attributable, in part, to immunosuppressive regimens used
Age
Children who receive allografts have higher frequency of PTLD
Likely related to higher frequency of EBV seronegativity at time of transplant
Patients who receive bone marrow/stem cell transplants have additional risk factors
HLA-mismatched allograft
Allograft that has been T cell depleted
Immunosuppressive therapy for graft vs. host disease
CLINICAL ISSUES
Epidemiology
Incidence
PTLD occurs in < 2-3% of all patients who receive organ allografts
Kidney: 1-3% of transplant patients
Liver: 1-3% of transplant patients
Heart: 1-6% of transplant patients
Heart-lung: 2-6% of transplant patients
Lung: 4-10% of transplant patients
Small intestine: ˜ 20% of transplant patients
Younger patients have higher incidence
Age
Predicted by age of population undergoing transplant
Gender
Predicted, in part, by underlying diseases of population undergoing transplant
Presentation
Highly variable; depends on
Organ(s) involved by PTLD
Histology of PTLD
Status of EBV infection
EBV(+) PTLDs usually occur within 5 years of transplant
Commonly arise within 1st year after transplant
EBV(-) cases occur a median of 50 months after transplantation
Constitutional symptoms are common
Lymphadenopathy; can be localized or systemic
Extranodal sites are commonly involved (up to 75% of cases)
Often involve gastrointestinal (GI) tract or brain
PTLD commonly involves allograft, but can be generalized
Associated with allograft failure
Most NK/T-cell PTLDs involve extranodal sites
Skin, blood, bone marrow, spleen, lung, GI tract
Bone marrow transplant recipients can develop generalized PTLD that mimics graft vs. host disease
± pancytopenia
Classical Hodgkin lymphoma type of PTLD is more common in patients with kidney or bone marrow/stem cell transplants
Natural History
Some monomorphic PTLDs may regress after discontinuation of immunosuppression
Relapse is common
Regression less likely if PTLD is EBV(-)
Most patients with monomorphic PTLDs require aggressive therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree