Post-transplant Lymphoproliferative Disorder, Early Lesions and Polymorphic
C. Cameron Yin, MD, PhD
Pei Lin, MD
Key Facts
Etiology/Pathogenesis
Epstein-Barr virus infection and impaired host immunosurveillance important
80% of all PTLDs are EBV(+)
Risk factors for developing PTLD
EBV seronegativity before transplant; age
Degree of overall immunosuppression
Types of immunosuppression or transplanted organ
Clinical Issues
Early lesions: Involve lymph nodes or Waldeyer ring
Reduction of immunosuppression often adequate
Polymorphic PTLDs: Involve lymph nodes &/or extranodal sites
Reduction of immunosuppression ± effective
Subset of patients require cytotoxic chemotherapy
Microscopic Pathology
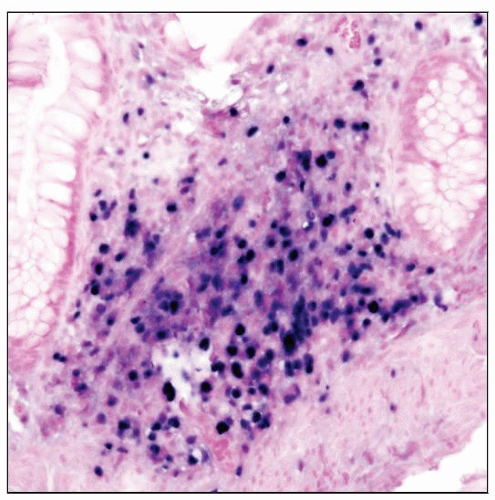
Plasmacytic hyperplasia
Preserved architecture; plasma cells, lymphocytes, and scattered immunoblasts
IM-like PTLD
Paracortical expansion by lymphocytes, plasma cells, and immunoblasts
Polymorphic PTLD
Effacement of architecture by plasma cells, lymphocytes, and immunoblasts; ± necrosis
Ancillary Tests
Early lesions
Polytypic plasma cells and B cells; EBV(+/-)
Polymorphic PTLD
˜ 50% monotypic &/or monoclonal; EBV(+/-)
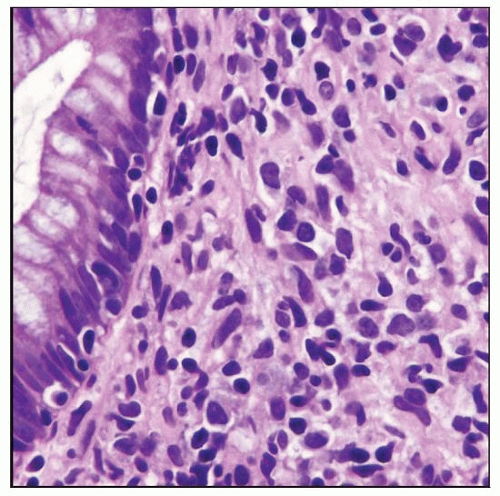 Polymorphic post-transplant lymphoproliferative disorder (PTLD) involving the rectum. Note the mixture of cell types present. |
TERMINOLOGY
Abbreviations
Post-transplant lymphoproliferative disorder (PTLD)
Definitions
Plasmacytic or lymphoid proliferations occurring as result of immunosuppression following solid organ or bone marrow transplantation
Early lesions are PTLDs characterized by architectural preservation of involved site
Polymorphic lesions are PTLDs that efface architecture but are morphologically heterogeneous and do not fulfill criteria for any known lymphoma type
ETIOLOGY/PATHOGENESIS
Infectious Agents
Epstein-Barr virus plays a central role
80% of all PTLDs are EBV(+)
Usually type A
Serum EBV antibody titers and blood EBV DNA load increase prior to onset of PTLD
Number of EBV(+) cytotoxic T cells drops prior to onset of PTLD
Treatment with EBV-specific T cells induces remission or responses in some patients
Analysis of EBV terminal repeat regions by Southern blot analysis has shown monoclonal form of virus
Indicates presence of EBV before monoclonal expansion began
EBV can transform germinal center (GC) B cells
Extended half-life of EBV-infected B cells increases likelihood of acquiring additional molecular aberrations that confer a growth advantage
Pathogenesis
Arise from GC or post-GC B cells
In solid organ allograft recipients, most PTLDs are of host origin
In bone marrow or stem cell allograft recipients, most PTLDs are of donor origin
Iatrogenically decreased host immunosurveillance
Risk factors for PTLD in general
EBV seronegativity before transplant
Degree of overall immunosuppression
Type of immunosuppression
Higher risk with tacrolimus, OKT3 monoclonal antibody, or antithymocyte globulin
Type of organs transplanted
Most common in intestinal and multiorgan transplant recipients
Lowest in renal transplant recipients
May be attributable to differences in immunosuppressive regimens used
Age
Pediatric patients have a higher incidence of PTLD
Most likely related to more frequent EBV seronegativity prior to transplant
Additional risk factors for patients who receive autologous bone marrow or stem cell transplants
HLA-mismatched allograft
T-cell-depleted allograft
Immunosuppressive therapy for graft vs. host disease
CLINICAL ISSUES
Epidemiology
Incidence
Frequency of PTLD is related to type of transplant and associated immunosuppression
Age
Predicted by age of patient population undergoing transplant
Younger patients are at increased risk of developing PTLD
Site
Early lesions
Primarily involving lymph nodes
Tonsils, adenoids
Polymorphic
Lymph nodes
Extranodal masses can occur
Presentation