FATTY ACIDS
These are straight-chain carbon compounds of varying lengths. They may be saturated, containing no double bonds, monounsaturated, with one double bond, or polyunsaturated, with more than one double bond (
Table 13.1).
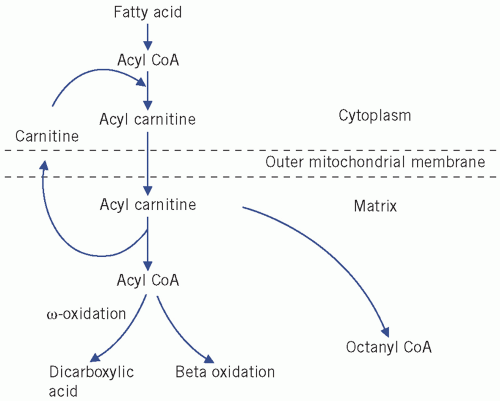
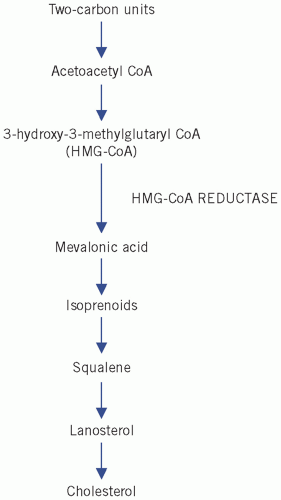
Fatty acids can esterify with glycerol to form triglycerides or be non-esterified (NEFAs) or free. Plasma NEFAs liberated from adipose tissue by lipase activity are transported to the liver and muscle mainly bound to albumin. The NEFAs provide a significant proportion of the energy requirements of the body. Summary diagrams of fatty acid synthesis and oxidation are shown in
Figures 13.2 and
13.3.
Triglycerides are transported from the intestine to various tissues, including the liver and adipose tissue, as lipoproteins. Following hydrolysis, fatty acids are taken up, re-esterified and stored as triglycerides. Plasma
triglyceride concentrations rise after a meal, unlike that of plasma cholesterol.
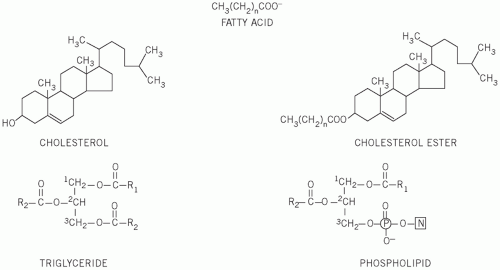
Phospholipids are complex lipids, similar in structure to triglycerides but containing phosphate and a nitrogenous base in place of one of the fatty acids. They fulfil an important structural role in cell membranes, and the phosphate group confers solubility on nonpolar lipids and cholesterol in lipoproteins.
A family of nuclear receptors that are activated by fatty acids – called peroxisome proliferator-activated receptors (PPARs) – has been described and implicated in insulin resistance and dyslipidaemia. The PPARs can be subdivided into α-PPARs, which are activated by fibrate drugs, and γ-PPARs, which are activated by thiazolidinedione drugs, for example pioglitazone or rosiglitazone.
LIPOPROTEINS
Because lipids are relatively insoluble in aqueous media, they are transported in body fluids as, often spherical,
soluble protein complexes called lipoproteins. Lipids can be derived from food (exogenous) or synthesized in the body (endogenous). The water-soluble (polar) groups of proteins, phospholipids and free cholesterol face outwards and surround an inner insoluble (nonpolar) core of triglyceride and cholesterol esters.
Lipoproteins are classified by their buoyant density, which inversely reflects their size. The greater the lipid to protein ratio, the larger their size and the lower the density. Lipoproteins can be classified into five main groups (
Table 13.2). The first three are triglyceride rich and, because of their large size, they scatter light, which can give plasma a turbid appearance (lipaemic) if present in high concentrations:
Chylomicrons are the largest and least dense lipoproteins and transport exogenous lipid from the intestine to all cells.
Very low-density lipoproteins (VLDLs) transport endogenous lipid from the liver to cells.
Intermediate-density lipoproteins (IDLs), which are transient and formed during the conversion of
VLDL to low-density lipoprotein (
LDL), are not normally present in plasma.
The other two lipoprotein classes contain mainly cholesterol and are smaller in size:
If a lipaemic plasma sample, for example after a meal, is left overnight at 4°C, the larger and less dense chylomicrons form a creamy layer on the surface. The smaller and denser
VLDL and
IDL particles do not rise, and the sample may appear diffusely turbid. The
LDL and
HDL particles do not contribute to this turbidity because they are small and do not scatter light. Fasting plasma from normal individuals contains only
VLDL,
LDL and
HDL particles.
In some cases of hyperlipidaemia, the lipoprotein patterns have been classified (Fredrickson’s classification) according to their electrophoretic mobility. Four principal bands are formed, based on their relative positions, by protein electrophoresis, namely α (
HDL), pre-β (
VLDL), β (
LDL) and chylomicrons (
Table 13.3).
Intermediate-density lipoproteins in excess may produce a broad β-band. Some individuals with hyperlipidaemia may show varying electrophoretic patterns at different times.
Ultracentrifugation (separation based upon particle buoyant density) or electrophoretic techniques are rarely used in routine clinical practice as these may require completed apparatus and experienced operators. Instead, the lipoprotein composition of plasma may be inferred from standard clinical laboratory lipid assays. As fasting plasma does not normally contain chylomicrons, the triglyceride content reflects
VLDL. Furthermore, generally about 70 per cent of plasma cholesterol is incorporated as
LDL and 20 per cent as
HDL. The latter particles, because of their high density, can be quantified by precipitation techniques that can assay their cholesterol content by subtraction, although direct
HDL assays are now often used.
The Friedewald equation enables plasma
LDL cholesterol concentration to be calculated and is often used in clinical laboratories:
This equation makes certain assumptions, namely that the patient is fasting and the plasma triglyceride concentration does not exceed 4.5 mmol/L (otherwise chylomicrons make the equation inaccurate).
There has been recent interest in the subdivision of
LDL particles into small dense
LDL2 and
LDL3, which appear to be more atherogenic and more easily oxidized than the larger
LDL1 particles. Additionally, another lipoprotein called lipoprotein (a), or Lp(a), has been found. This is similar in lipid composition to
LDL but has a higher protein content. One of its proteins, called apolipoprotein (a), shows homology to plasminogen and may disrupt fibrinolysis, thus evoking a thrombotic tendency. The plasma concentration of Lp(a) is normally less than 0.30 g/L and it is thought to be an independent cardiovascular risk factor.
The proteins associated with lipoproteins are called apolipoproteins (
apo). ApoA (mainly apoA1 and apoA
2) is the major group associated with
HDL particles. The apoB series (apoB
100) is predominantly found with
LDL particles and is the ligand for the
LDL receptor. Low-density lipoprotein has one molecule of apoB
100 per particle. Some reports have suggested that the plasma apoA
1 to apoB ratio may be a useful measure of cardiovascular risk (increased if the ratio is less than 1) and it is not significantly influenced by the fasting status of the patient. The apoC series is particularly important in triglyceride metabolism and, with the apoE series, freely interchanges between various lipoproteins. Some of the functions of these apolipoproteins are described in
Table 13.4.
Lipoprotein-associated phospholipase A
2 [also called platelet-activating factor acetylhydrolase (PAFAH)] is present mainly on
LDL and to a lesser degree
HDL. It is produced by inflammatory cells and is involved in atherosclerosis formation and levels are associated with increased risk of coronary artery disease and stroke.
??
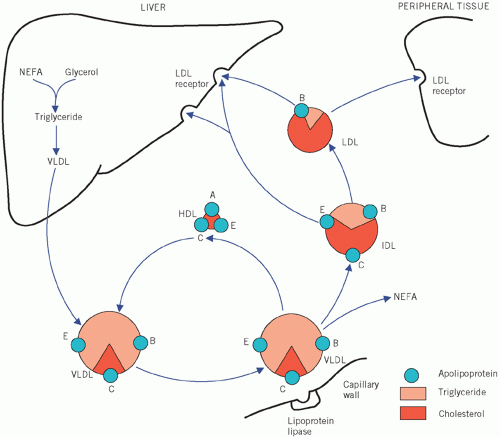
from chylomicron remnants via the exogenous pathway or synthesized locally. These lipids are transported from the liver as
VLDL.
Very low-density lipoprotein is a large triglyceriderich particle consisting also of apoB
100, apoC and apoE. Following hepatic secretion, it incorporates additional apoC from
HDL particles within the circulation. Like chylomicrons,
VLDL is hydrolysed by lipoprotein lipase in the peripheral tissues, albeit more slowly. The resulting
VLDL remnant or
IDL contains cholesterol and triglyceride as well as apoB and apoE and is rapidly taken up by the liver or converted by the action of hepatic lipase to
LDL by losing apoE and triglyceride.
Low-density lipoprotein is a small cholesterol-rich lipoprotein containing only apoB. It represents about 70 per cent of the total plasma cholesterol concentration. It can be taken up by most cells, although mainly the liver by the
LDL or B/E receptor which recognizes and binds apoB
100. Within the cell, the
LDL particles are broken down by lysosomes, releasing cholesterol. This cholesterol can be incorporated into cell membranes or in specific tissues such as the adrenal cortex or gonads and utilized in steroid synthesis.
Most cells are able to synthesize cholesterol, but, to avoid intracellular accumulation, there is a feedback control system reducing the rate of synthesis of the
LDL receptors. Although most of the plasma
LDL is removed by
LDL receptors, if the plasma cholesterol concentration is excessive,
LDL particles, by virtue of their small size, can infiltrate tissues by passive diffusion and can even cause damage, as in atheroma formation within arterial walls. An alternative route of removal of
LDL is via the reticuloendothelial system, collectively termed the scavenger cell pathway, which recognizes only chemically modified
LDL, for example oxidized
LDL.
The liver has a central role in cholesterol metabolism: