INTRODUCTION
Picornaviruses represent a very large virus family with respect to the number of members but one of the smallest in terms of virion size and genetic complexity. They include two major groups of human pathogens: enteroviruses and rhinoviruses. Enteroviruses are transient inhabitants of the human alimentary tract and may be isolated from the throat or lower intestine. Rhinoviruses are associated with the respiratory tract and isolated chiefly from the nose and throat. Less common picornaviruses associated with human illness include hepatitis A virus, parechovirus, cardiovirus, and Aichi virus. Several genera of picornaviruses are also associated with animal, plant, and insect disease.
Many picornaviruses cause diseases in humans ranging from severe paralysis to aseptic meningitis, pleurodynia, myocarditis, vesicular and exanthematous skin lesions, mucocutaneous lesions, respiratory illnesses, undifferentiated febrile illness, conjunctivitis, and severe generalized disease of infants. However, subclinical infection is far more common than clinically manifest disease. Etiology is difficult to establish because different viruses may produce the same syndrome, the same picornavirus may cause more than a single syndrome, and some clinical symptoms cannot be distinguished from those caused by other types of viruses. The most serious disease caused by any enterovirus is poliomyelitis.
A worldwide effort is making progress toward the goal of total eradication of poliomyelitis.
PROPERTIES OF PICORNAVIRUSES
Important properties of picornaviruses are shown in Table 36-1.
| Virion: Icosahedral, 28–30 nm in diameter, contains 60 subunits |
| Composition: RNA (30%), protein (70%) |
| Genome: Single-stranded RNA, linear, positive sense, 7.2–8.4 kb in size, molecular weight 2.5 million, infectious, contains genome-linked protein (VPg) |
| Proteins: Four major polypeptides cleaved from a large precursor polyprotein. Surface capsid proteins VP1 and VP3 are major antibody-binding sites. VP4 is an internal protein. |
| Envelope: None |
| Replication: Cytoplasm |
| Outstanding characteristic: Family is made up of many enterovirus and rhinovirus types that infect humans and lower animals, causing various illnesses ranging from poliomyelitis to aseptic meningitis to the common cold. |
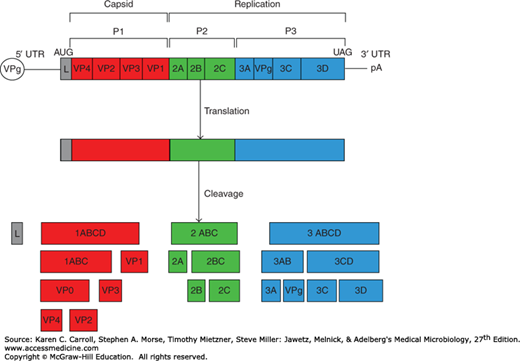
The virion of enteroviruses and rhinoviruses consists of a capsid shell of 60 subunits, each of four proteins (VP1–VP4) arranged with icosahedral symmetry around a genome made up of a single strand of positive-sense RNA (Figure 36-1). Parechoviruses are similar except that their capsids contain only three proteins because VP0 does not get cleaved into VP2 and VP4.
FIGURE 36-1
Structure of a typical picornavirus. A: Exploded diagram showing internal location of the RNA genome surrounded by capsid composed of pentamers of proteins VP1, VP2, VP3, and VP4. Note the “canyon“ depression surrounding the vertex of the pentamer. B: Binding of cellular receptor to the floor of the canyon. The major rhinovirus receptor (intercellular adhesion molecule-1 [ICAM-1]) has a diameter roughly half that of an immunoglobulin G (IgG) antibody molecule. C: Location of a drug-binding site in VP1 of a rhinovirus. The antiviral drug shown, WIN 52084, prevents viral attachment by deforming part of the canyon floor. (Reproduced with permission from Rueckert RR: Picornaviridae: The viruses and their replication. In Fields BN, Knipe DM, Howley PM [editors-in-chief]. Fields Virology, 3rd ed. Lippincott-Raven, 1996.)
By means of x-ray diffraction studies, the molecular structures of poliovirus and rhinovirus have been determined. The three largest viral proteins—VP1, VP2, and VP3—have a very similar core structure in which the peptide backbone of the protein loops back on itself to form a barrel of eight strands held together by hydrogen bonds (the β barrel). The amino acid chain between the β barrel and the amino and carboxyl terminal portions of the protein contains a series of loops. These loops include the main antigenic sites that are found on the surface of the virion and are involved in the neutralization of viral infection.
There is a prominent cleft or canyon around each pentameric vertex on the surface of the virus particle. The receptor-binding site used to attach the virion to a host cell is thought to be located near the floor of the canyon. This location would presumably protect the crucial cell attachment site from structural variation influenced by antibody selection in hosts because the canyon is too narrow to permit deep penetration of antibody molecules (Figure 36-1).
The genome RNA ranges in size from 7.2 kb (human rhinovirus) to 7.4 kb (poliovirus, hepatitis A virus) to 8.4 kb (aphthovirus). The organization of the genome is similar for all (Figure 36-2). The genome is polyadenylated at the 3′ end and has a small viral-coded protein (VPg) covalently bound to the 5′ end. The positive-sense genomic RNA is infectious.
FIGURE 36-2
Organization and expression of the picornavirus genome. The viral genomic RNA has genome-linked VPg protein at the 5′ end and is polyadenylated at the 3′ terminus. L specifies a leader protein found in cardioviruses and aphthoviruses but not in enteroviruses, human rhinoviruses, or human hepatitis virus A. The plus-sense single-stranded RNA genome is translated into a single polyprotein. The P1 domain (red) encodes capsid proteins, and the P2 (green) and P3 (blue) domains encode noncapsid proteins used for protein processing and replication. Cleavage of the polyprotein is accomplished by virus-coded proteinases 2A and 3C. Protein 2A performs early cleavages of the polyprotein, and all other cleavages are performed by proteinase 3C. (Reproduced with permission from Kerkvliet J, Edukulla R, Rodriguez M: Novel roles of the picornaviral 3D polymerase in viral pathogenesis. Adv Virol 2010;368068. Copyright © 2010 Jason Kerkvliet et al.)
Whereas enteroviruses are stable at acid pH (3.0–5.0) for 1–3 hours, rhinoviruses are acid labile. Enteroviruses and some rhinoviruses are stabilized by magnesium chloride against thermal inactivation. Enteroviruses have a buoyant density in cesium chloride of about 1.34 g/mL; human rhinoviruses, about 1.4 g/mL.
The Picornaviridae family contains 12 genera, including Enterovirus (enteroviruses and rhinoviruses), Hepatovirus (hepatitis A virus), Kobuvirus (Aichi virus), Parechovirus (parechoviruses), Cardiovirus (cardioviruses), and Aphthovirus (foot-and-mouth disease viruses). The first five groups contain important human pathogens. Rhinoviruses historically were placed in a separate genus but are now considered to be members of the Enterovirus genus.
Enteroviruses of human origin are subdivided into seven species (human enterovirus A–D and human rhinovirus A–C) based mainly on sequence analyses. The former taxonomy for these viruses included the following: (1) polioviruses, types 1–3; (2) coxsackieviruses of group A, types 1–24 (there is no type 15, 18, or 23); (3) coxsackieviruses of group B, types 1–6; (4) echoviruses, types 1–33 (no types 8, 10, 22, 23, 28, or 34); and (5) enteroviruses, types 68–116 (no type 72) (Table 36-2). Since 1969, new enterovirus types have been assigned enterovirus type numbers rather than being subclassified as coxsackieviruses or echoviruses. The vernacular names of the previously identified enteroviruses have been retained. The coxsackie A viruses fall into human enterovirus species HEV-A and HEV-C and coxsackie B viruses and echoviruses into HEV-B.
| Property | Human Enteroviruses A–D | Human Rhinoviruses A–Cc | Human Parechovirusesd | ||||
|---|---|---|---|---|---|---|---|
| Polio | Coxsackie Aa | Coxsackie B | Echoa | Enterob | |||
| Serotypes | 1–3 | 1–24 | 1–6 | 1–33 | 68–116 | >150 | 1–14 |
| Acid pH (pH 3.0) | Stable | Stable | Stable | Stable | Stable | Labile | Stable |
| Density (g/mL) | 1.34 | 1.34 | 1.34 | 1.34 | 1.34 | 1.4 | |
| Optimal temperature for growth (°C) | 37 | 37 | 37 | 37 | 37 | 33 | 37 |
| Common sites of isolation from humans | |||||||
| Nose | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Throat | + | + | + | + | + | + | |
| Lower intestine | + | + | + | + | + | 0 | + |
| Infect newborn micee | 0 | + | + | 0 | 0 | ||
Human rhinoviruses include more than 100 antigenic types and fall into human rhinovirus (HRV) species A, B, and C. Rhinoviruses of other host species include those of horses and cattle.
Hepatitis A virus was originally classified as enterovirus type 72 but is now assigned to a separate genus. It is described in Chapter 35.
Parechoviruses, previously classified as echoviruses 22 and 23, were found to differ significantly from the enteroviruses in both biologic properties and molecular characteristics and were placed into a new genus, Parechovirus.
Other picornaviruses are foot-and-mouth disease virus of cattle (Aphthovirus) and encephalomyocarditis virus of rodents (Cardiovirus).
The host range of picornaviruses varies greatly from one type to the next and even among strains of the same type. Many enteroviruses (polioviruses, echoviruses, some coxsackieviruses) can be grown at 37°C in human and monkey cells; most rhinovirus strains can be recovered only in human cells at 33°C. Coxsackieviruses are pathogenic for newborn mice.
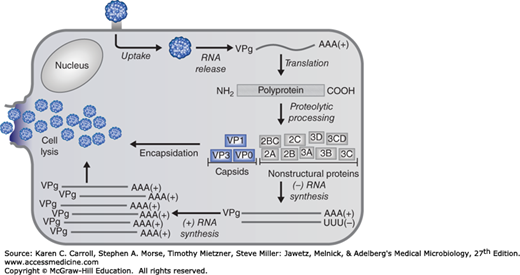
The picornavirus replication cycle occurs in the cytoplasm of cells (Figure 36-3). First, the virion attaches to a specific receptor in the plasma membrane. The receptors for poliovirus and human rhinovirus are members of the immunoglobulin gene superfamily, which includes antibodies and some cell surface adhesion molecules. In contrast, echoviruses recognize a member of the integrin adhesion superfamily. Not all rhinoviruses or echoviruses use the same cellular receptor. The viruses that cause hand-foot-and-mouth disease (enterovirus 71 and coxsackievirus A16) can both use two receptors, SCARB2 and PSGL1. Receptor binding triggers a conformational change in the virion which results in release of the viral RNA into the cell cytosol. VPg is removed from the viral RNA as it associates with ribosomes. Translation occurs via a cap-independent mechanism, using the internal ribosome entry site (IRES) downstream from the 5′ end of the viral genome. This bypasses the need for intact cellular initiation factor complex (eIF4F), which is required by many capped cellular mRNAs. eIF4 is often cleaved by a viral protease, leading to shut-off of host protein synthesis and preferential translation of viral RNAs.
The infecting viral RNA is translated into a polyprotein that contains both coat proteins and essential replication proteins. This polyprotein is rapidly cleaved into fragments by proteinases encoded in the polyprotein (see Figure 36-2). Synthesis of new viral RNA cannot begin until the virus-coded replication proteins, including an RNA-dependent RNA polymerase, are produced. The infecting viral RNA strand is copied, and that complementary strand serves as template for the synthesis of new plus strands. Many plus strands are generated from each minus-strand template. Some new plus strands are recycled as templates to amplify the pool of progeny RNA; many plus strands get packaged into virions.
Maturation involves several cleavage events. Coat precursor protein P1 (see Figure 36-2) is cleaved to form aggregates of VP0, VP3, and VP1. When an adequate concentration is reached, these “protomers” assemble into pentamers that package plus-stranded VPg-RNA to form “provirions.” The provirions are not infectious until a final cleavage changes VP0 to VP4 and VP2. The mature virus particles are released when the host cell disintegrates. The multiplication cycle for most picornaviruses takes 5–10 hours.
ENTEROVIRUS GROUP
Poliomyelitis is an acute infectious disease that in its serious form affects the central nervous system (CNS). The destruction of motor neurons in the spinal cord results in flaccid paralysis. However, most poliovirus infections are subclinical.
Poliovirus has served as a model enterovirus in many laboratory studies of the molecular biology of picornavirus replication.
Poliovirus particles are typical enteroviruses (see earlier). They are inactivated when heated at 55°C for 30 minutes, but Mg2+, 1 mol/L, prevents this inactivation. Whereas purified poliovirus is inactivated by a chlorine concentration of 0.1 ppm, much higher concentrations of chlorine are required to disinfect sewage containing virus in fecal suspensions and in the presence of other organic matter. Polioviruses are not affected by ether or sodium deoxycholate.
Polioviruses have a very restricted host range. Most strains will infect monkeys when inoculated directly into the brain or spinal cord. Chimpanzees and cynomolgus monkeys can also be infected by the oral route; in chimpanzees, the infection is usually asymptomatic and the animals become intestinal carriers of the virus.
Most strains can be grown in primary or continuous cell line cultures derived from a variety of human tissues or from monkey kidney, testis, or muscle but not from tissues of lower animals.
Poliovirus requires a primate-specific membrane receptor for infection, and the absence of this receptor on the surface of nonprimate cells makes them virus resistant. This restriction can be overcome by transfection of infectious poliovirus RNA into resistant cells. Introduction of the viral receptor gene converts resistant cells to susceptible cells. Transgenic mice harboring the primate receptor gene have been developed; they are susceptible to human polioviruses.
There are three antigenic types of polioviruses based on epitopes found in the VP1, VP2, and VP3 proteins.
The mouth is the portal of entry of the virus, and primary multiplication takes place in the oropharynx or intestine. The virus is regularly present in the throat and in the stools before the onset of illness. One week after infection, there is little virus in the throat, but virus continues to be excreted in the stools for several weeks even though high antibody levels are present in the blood.
The virus may be found in the blood of patients with nonparalytic poliomyelitis. Antibodies to the virus appear early in the disease, usually before paralysis occurs.
It is believed that the virus first multiplies in the tonsils, the lymph nodes of the neck, Peyer’s patches, and the small intestine. The CNS may then be invaded by way of the circulating blood.
Poliovirus can spread along axons of peripheral nerves to the CNS, where it continues to progress along the fibers of the lower motor neurons to increasingly involve the spinal cord or the brain. Poliovirus invades certain types of nerve cells, and in the process of its intracellular multiplication, it may damage or completely destroy these cells.
Poliovirus does not multiply in muscle in vivo. The changes that occur in peripheral nerves and voluntary muscles are secondary to the destruction of nerve cells. Some cells that lose their function may recover completely. Inflammation occurs secondary to the attack on the nerve cells.
In addition to pathologic changes in the nervous system, there may be myocarditis, lymphatic hyperplasia, and ulceration of Peyer’s patches.
When an individual susceptible to infection is exposed to the virus, the response ranges from inapparent infection without symptoms to a mild febrile illness to severe and permanent paralysis. Most infections are subclinical; only about 1% of infections result in clinical illness.
The incubation period is usually 7–14 days, but it may range from 3 to 35 days.
This is the most common form of disease. The patient has only a minor illness, characterized by fever, malaise, drowsiness, headache, nausea, vomiting, constipation, and sore throat in various combinations. Recovery occurs in a few days.
In addition to the symptoms and signs listed in the preceding paragraph, the patient with the nonparalytic form has stiffness and pain in the back and neck. The disease lasts 2–10 days, and recovery is rapid and complete. Poliovirus is only one of many viruses that produce aseptic meningitis. In a small percentage of cases, the disease advances to paralysis.
The predominating complaint is flaccid paralysis resulting from lower motor neuron damage. However, incoordination secondary to brain stem invasion and painful spasms of nonparalyzed muscles may also occur. The amount of damage varies greatly. Maximal recovery usually occurs within 6 months, with residual paralysis lasting much longer.
A recrudescence of paralysis and muscle wasting has been observed in individuals decades after their experience with paralytic poliomyelitis. Although progressive postpoliomyelitis muscle atrophy is rare, it is a specific syndrome. It does not appear to be a consequence of persistent infection but rather a result of physiologic and aging changes in paralytic patients already burdened by loss of neuromuscular functions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree