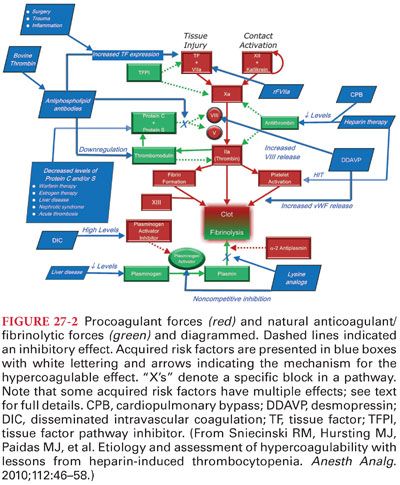
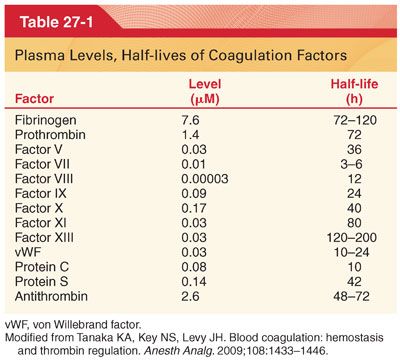
II. Hemostasis and History (see Figs. 27-1 and 27-2) (Table 27-1)
A. Initiation of Coagulation. Initiation of coagulation by procoagulant activities has been traditionally separated into extrinsic, intrinsic, and common pathways (a better understanding of the complex interactions has created a better conceptual integration of these pathways). Following tissue injury and vascular endothelial disruption, activation of hemostasis occurs by tissue factor (TF) expression on the subendothelial vascular basement of the blood vessel. As part of the activation, there are also checks and balances in the system to prevent an over exuberant prothrombotic effect from occurring.
B. Propagation of Coagulation. Platelets further amplify or potentially initiate clot formation at the site of vascular injury. Inflammatory cells all contain adhesion molecules to facilitate binding in the rapid flow of blood vessels. Thrombin activation of platelets further amplifies clot formation by multiple mechanisms. In the absence of factor VIIIa or factor IXa, as is clinically observed in hemophilia A or B, the initiation of coagulation is normal, but amplification/propagation is altered. Patients with hemophilia clot, but they develop bleeding in muscle and joints due to low TF expression.
1. Tissue Factor, Thrombin, and Fibrin(ogen) in Clot Formation and Stability. When generated, thrombin facilitates the proteolytic conversion of circulating, soluble fibrinogen to an insoluble fibrin meshwork (provides structural support to the clot).
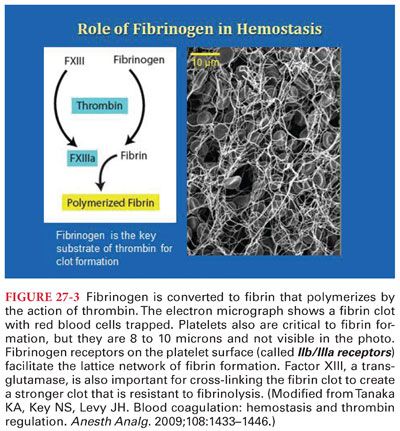
2. Role of Fibrinogen (Fig. 27-3). Fibrinogen is a critical protein for clot formation and has a critical role in hemostasis.
a. Of all the coagulation factors, fibrinogen circulates at the highest concentration (7.6 μM, ~200 to 400 mg/dL). In pregnancy and during acute inflammatory responses that often occur postoperatively, fibrinogen is an acute-phase reactant.
b. In patients with hemophilia and other bleeding abnormalities, lower levels of thrombin generation occur and as a result clot formation is altered, thus hemophiliacs commonly bleed into joints.
3. Critical factor levels for hemostasis. A critical question is what levels of fibrinogen, platelets, and other coagulation proteins are necessary to optimize hemostasis in the surgical patients (many recommend treating fibrinogen levels if they have decreased below <1.0 g/L [100 mg/dL]).
4. Role of Factor XIII. Factor XIII plays a major role in the terminal phase of the clotting cascade that promotes formation of cross-linked fibrin polymers and generation of a stable hemostatic plug.
5. Role of Platelets and von Willebrand Factor. Platelets adhere to sites of vascular injury and to each other by direct and indirect effects that are part of a complex cellular mechanism required for hemostasis.
a. Following vascular injury, the subendothelial surface is exposed which then binds to vWF that is synthesized in the endothelium and critical for platelet adhesion in arteries and arterioles that have high shear rates.
b. Platelets provide a catalytic membrane surface for further thrombin generation and clot formation.
c. When activated, platelets may also form occlusive thrombi in cardiovascular diseases that result in myocardial infarction, stroke, or other acute ischemic syndromes of other organs.
C. Endothelial Regulation of Coagulation (see Fig. 27-2). The vascular endothelium provides an extensive interface that is critical for both anticoagulant and procoagulant functions as shown.
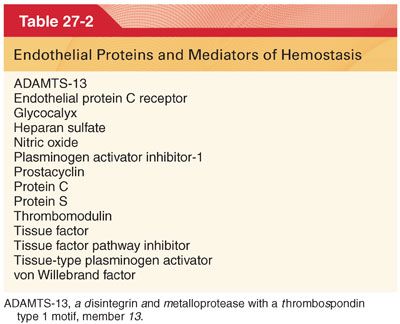
1. Increased shear forces and flow across the endothelium release important anticoagulation agents (Table 27-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


