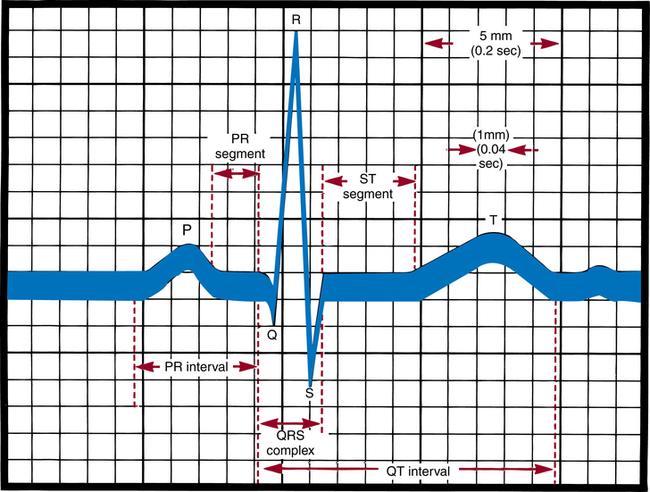
Chapter 27 After studying this chapter, the learner will be able to: • Identify pertinent body systems that should be monitored during a surgical procedure. • Discuss the differences between invasive and noninvasive means of patient monitoring. • Discuss why personnel monitoring a patient should be knowledgeable about the monitoring devices used. • Describe how monitoring parameters provide information about interrelated body systems. The development of successful controllable anesthesia has made modern surgery possible.5,10,14 Because anesthesia is an adjunct to most surgical procedures, familiarity with various anesthetic agents, their interaction with certain drugs, and their potential hazards is a necessity. The perioperative nurse responsible for patient monitoring may detect the onset of complications and help avert an undesired outcome. Working with anesthesia providers in the perioperative environment gives the learner an unparalleled opportunity to master immediate resuscitative measures and their effectiveness, and an understanding of the care of unconscious and critically ill patients. For example, the learner daily observes endotracheal intubation, ventilatory control, insertion of arterial and venous cannulas, fluid replacement, and sophisticated hemodynamic monitoring.14 Invasive hemodynamic monitoring uses basic physiologic principles to detect and treat a wide variety of abnormalities. Its purpose is to avoid problems in high-risk patients and to accurately diagnose and treat patients with established life-threatening disorders.14 Hemodynamic monitoring involves direct intravascular measurements and assessments by means of indwelling catheters connected to transducers and monitors. • ABGs and pressure via a single-lumen intraarterial catheter • Central venous pressure via a central venous, Hickman, or Broviac catheter • Pulmonary artery pressures via a pulmonary artery or Swan-Ganz catheter 1. Explain the procedure and reassure the patient. If the patient will be awake, sedation may be ordered. 2. Document the patient’s vital signs and pulse distal to the selected insertion site. If the pulse weakens after cannulation, circulation may be inadequate in an extremity and the catheter may need to be removed. 3. Position the patient as appropriate. a. For radial artery cannulation, affix the forearm to an armboard with the hand supinated and wrist dorsiflexed to an angle of 50 to 60 degrees over a towel. Avoid extreme dorsiflexion; this can obliterate the pulse. Tape the thumb to the armboard to stabilize the artery at the wrist. b. For subclavian or jugular vein insertion, place the patient in a 25-degree to 30-degree Trendelenburg’s position to reduce the potential for air embolism. Elevate the right scapular area with padding or a rolled towel underneath the shoulders to allow the physician to identify anatomic landmarks and locate the vein more easily. Turn the patient’s head away from the insertion site. 4. Prepare the skin per routine procedure. Wearing sterile gloves, the anesthesia provider then drapes the area. Warn patients if their face will be covered. 5. Inform patients, if awake, that they may have a burning sensation for a few seconds when the local anesthetic is injected before the area becomes numb. Explain that pressure, but not pain, may be felt during insertion. The skin and subcutaneous tissues are infiltrated with a local anesthetic because the skin is incised to facilitate entrance of the catheter. A cutdown, or opening of the skin and tissues to access a vein may be necessary. 6. Assist the anesthesia provider as appropriate. Be familiar with and follow manufacturer directions for the brand of catheter and monitoring equipment used. 7. Make sure the connections between the catheter and infusion line are secure after the catheter has been inserted and properly placed. The catheter is sutured in place with a synthetic monofilament suture to prevent inadvertent advancement or removal and is taped to the skin. Lumens on the three-way stopcock and catheter may be capped to prevent fibrin deposits and retrograde contamination. 8. Connect the catheter line to the transducer or monitor, and take baseline pressure readings. 9. Dress the puncture site. An antibacterial ointment may be put around the site. Tape must not apply pressure directly over the insertion site or catheter. A transparent dressing is preferable. The catheter beneath it must not be bent or curled. 10. Take the patient’s vital signs. Use a sphygmomanometer with the blood pressure cuff on the opposite arm from the insertion site, and check the blood pressure to compare with the monitor’s pressure reading to verify the monitor’s accuracy. The monitor may read higher systolic and lower diastolic pressures than the blood pressure cuff readings. 11. Document the procedure and initial readings. Include the insertion site; type and gauge of catheter; type of infusion solution and amount of heparin, if added; flow rate and pressure; pulse before and after insertion; tolerance of the procedure; color, sensation, and warmth of the area distal to the insertion site; time of insertion; and names of insertion team members. 1. Wear sterile gloves. A sterile heparinized syringe is used to prevent the blood samples from clotting. To heparinize, draw 1 mL of aqueous heparin 1:1000 into a 10-mL syringe. While rotating the barrel, pull the plunger back beyond the 7-mL calibration. With the syringe in an upright position, slowly eject the heparin and air bubbles while rotating the barrel. 2. Attach a sterile 5-mL syringe to the stopcock lumen going to outside air. Turn off (close) the infusion lumen. This automatically opens the line between the patient and the syringe. Aspirate to clear the line of fluid, and close the lumen to the patient. Discard this diluted sample. 3. Quickly attach the sterile heparinized syringe to a lumen to outside air, and open the lumen to the patient. This closes the lumen to the infusion, permitting aspiration of undiluted blood for analysis. Arterial pressure forces blood into the syringe. Withdraw 3 to 5 mL of blood. Hold the barrel, and the plunger, of the syringe to avoid their separation. Cap the syringe for placement in a properly labeled specimen bag. 4. Close the lumen to the patient, and flush the line and stopcock by letting the infusion solution run through them to prevent clot formation inside the catheter wall or stopcock, which could result in arterial embolization. 5. Close and recap the lumen to outside air (being careful not to contaminate the cap), thereby restarting the infusion to the patient. Regulate the infusion rate with the clamp on the infusion tubing. 6. If air bubbles are in the syringe, remove them. Send the samples immediately to the laboratory. If more than 10 minutes elapses between blood drawing and analysis, the analysis cannot be considered accurate. In the event of delay, the syringe with blood should be immersed in ice immediately and refrigerated at near-freezing temperature. Iced specimen bags may be used. 7. Attach the appropriate laboratory slips that include information such as the patient’s name and location, the time and date, and whether the patient is receiving oxygen supplement or breathing room air. Noninvasive methods can be used to monitor some cardiopulmonary and neural functions and to determine body temperature and urinary output (Box 27-1). Both noninvasive and invasive techniques are used for monitoring hemodynamic parameters to show minute-to-minute changes in physiologic variables. Normal ranges of hemodynamic parameters are given in Table 27-1. TABLE 27-1 Hemodynamic Monitoring Parameters Every heartbeat depends on the electrical process of polarization. Muscles in the heart wall are alternately stimulated and relaxed. An ECG is a recording of electrical forces produced by the heart and translated as waveforms (Fig. 27-1). It shows changes in rhythm, rate, and conduction, such as dysrhythmias, appearance of premature beats, and block of impulses. An ECG does not provide an index of cardiac output (CO). Cardiac monitoring has become standard procedure in the OR and postanesthesia care unit (PACU).2,3 The ECG tracing becomes a flat line when heart action ceases, but preceding tracings may define the type of cardiac arrest, which is of value in treatment. It is beyond the scope of this text to describe normal and abnormal cardiac rhythms interpreted by the ECG. However, perioperative nurses who monitor patients under local anesthesia should become familiar with them. Box 27-2 shows the characteristics of sinus rhythm, and Figure 27-2 shows an example of sinus rhythm in each of 12 leads.
Physiologic maintenance and monitoring of the perioperative patient
Monitoring physiologic functions
Invasive hemodynamic monitoring
Intravascular catheters
Catheter insertion.
Drawing blood samples.
Physiologic parameters monitored
Parameter
Abbreviation
Normal Range for Adults
Arterial oxygen content
Cao2
17-20 mL/dL blood
Blood pressure
BP
Systolic 90-130 mm HgDiastolic 60-85 mm Hg
Cardiac index
CI
2.8-4.2 L/min/m2
Cardiac output
CO
4-8 L/min
Central venous pressure
CVP
2-8 mm Hg, 3-10 cm H2O
Cerebral perfusion pressure
CPP
80-100 mm Hg
Coronary perfusion pressure
CPP
60-80 mm Hg
Ejection fraction
EF
60%-70%
Glomerular filtration rate
GFR
80-120 mL/min
Heart rate
HR
60-100 beats/min
Intracranial pressure
ICP
0-15 mm Hg
Left ventricular end-diastolic pressure
LVEDP
8-12 mm Hg
Mean arterial pressure
MAP
70-105 mm Hg
Mean pulmonary artery pressure
MPAP
9-19 mm Hg
Oxygen saturation in arterial blood
Sao2
95%-97.5%
Oxygen saturation in mixed venous blood
Svo2
75%
Partial pressure of carbon dioxide in arterial blood
Paco2
34-45 mm Hg (torr)
Partial pressure of oxygen in arterial blood
Pao2
80-100 mm Hg (torr)
Partial pressure of oxygen in venous blood
Pvo2
40 mm Hg (torr)
Pulmonary artery pressure
PAP
Systolic: 15-25 mm HgDiastolic: 8-15 mm Hg
Pulmonary capillary wedge pressure
PCWP
6-12 mm Hg
Right atrial pressure
RAP
3-6 mm Hg
Right ventricular pressure
RVP
Systolic: 15-25 mm HgDiastolic: 0-5 mm Hg
Stroke volume
SV
60-130 mL/beat
Systemic vascular resistance
SVR
800-1600 dyne/sec/cm
Total blood volume
TBV
8.5%-9% of body weight in kg
Venous oxygen content
Cvo2
15 mL/dL blood
Electrocardiogram

 Website
Website