Phyllodes Tumor
Key Facts
Terminology
Derived from the Greek word “phyllos” meaning “leaf”
Biphasic tumor consisting of epithelial-lined clefts separating areas of cellular stroma, creating leaf-like fronds
Clinical Issues
Uncommon: < 1% of all breast tumors
Peak age range is 35 -55
More common in Asian populations (˜ 7% of breast tumors)
> 90% of PTs follow benign clinical course and are adequately treated by surgical resection
In rare cases (< 5%), distant metastases occur and usually result in death of patient
Microscopic Pathology
Evaluation of stroma is used to classify PT into 3 grades
Margins can be difficult to evaluate, particularly in reexcision specimens
Recurrent PT can progress to a higher grade and acquire additional genetic changes
Metastases consist only of the stromal component
Bone and lung are most common sites
Top Differential Diagnoses
Fibroadenoma with cellular stroma
Spindle cell carcinoma
Primary sarcoma of breast
Fibromatosis
Metastatic spindle cell carcinoma
TERMINOLOGY
Abbreviations
Phyllodes tumor (PT)
Synonyms
Cystosarcoma phyllodes
Periductal stromal sarcoma
Definitions
Biphasic tumor consisting of neoplastic intralobular-type stromal cells and benign epithelial cells
ETIOLOGY/PATHOGENESIS
Cell of Origin
During development of embryo, 1st breast cells to differentiate are stromal cells
Stroma induces downgrowth of overlying epithelium to form primitive ducts
This molecular cross-talk continues throughout development
Neoplastic stromal cells of PT arise from fibroblasts of specialized intralobular stroma
Fibroadenomas (FAs) are closely related tumors that also arise from same cells
Some FAs are hyperplasias and some neoplasias
FA and low-grade PT are on a spectrum of increasing autonomous growth of stromal cells
Some PT may arise from a preexisting FA
Associated epithelial cells are benign
Epithelial cells are stimulated to proliferate by stromal cells
In some PTs, epithelial cells have some of the same genetic changes as stromal cells
May reflect fact that lobules are derived from a clone of cells
In other cases, epithelial cells are polyclonal
With very rare exceptions, only stromal cells progress to malignancy
With increasing autonomy and growth, stromal cells outgrow epithelial cells
High-grade PT may consist almost entirely of stromal cells
Only stromal cells are present in distant metastases
DNA Changes
Number of chromosomal changes increases with grade of PT
Most common changes are gain of 1q and loss of 13, 7p12, 3p24, 10p12, and 9p21
Changes are very variable from tumor to tumor
Changes are also very variable within tumors
Suggests that many subclones with different genetic changes arise during progression
Loss of heterozygosity (LOH) is very low in FAs, more common in low- and intermediate-grade PT, and most common in high-grade PT
Recurrent PT often gains genetic changes
Correlates with increase in histologic grade in ˜ 1/3 of cases
Gene Expression Profiling
Patterns of expression vary by PT grade
Supports separation into 3 groups
Major categories of changes are in genes related to proliferation and stromal/epithelial interactions
CLINICAL ISSUES
Epidemiology
Presentation
Most common presentation is as palpable painless mass
Less commonly detected as a density on mammographic screening
Treatment
Surgical approaches
Complete excision of all PTs recommended
Incomplete excision increases risk for local recurrence
Recurrence rates increase from low-grade to high-grade PT; majority occur in 1st 2 years
Lower rates of recurrence after mastectomy
Adjuvant therapy
Chemotherapy has not been shown to be effective
Radiation
May reduce local recurrence rate
Not generally used for initial treatment
Prognosis
Difficult to predict
Rare and difficult to study
Large series from referral centers may not be representative of prognosis in general
Treatment is not standardized
Outcome can be predicted to some extent by grade of tumor
Low-grade PT (also termed “benign”) ˜ 60% of PT
Features overlap with cellular FA
˜ 4-10% risk of local recurrence; lower with more extensive surgery
< 1% risk of distant metastasis; reported to occur in large series, but these cases have never been described in detail
PT can recur at a higher grade; this may explain rare cases with metastasis
Intermediate-grade PT (also termed “borderline”) ˜ 10-20% of PT
˜ 20% risk of local recurrence; lower with more extensive surgery
< 10% risk of distant metastasis
High-grade PT (also termed “malignant”) ˜ 10-20% of PT
˜ 20% risk of local recurrence; lower with more extensive surgery
˜ 30% risk of distant metastasis
Core Needle Biopsy
It can be difficult to distinguish PT from FA on core needle biopsies
PT can be heterogeneous with some areas having appearance of FA
History of recent increase in size favors PT unless patient is pregnant
Features favoring PT on core needle biopsy
Markedly cellular stroma
Invasive border
Stromal overgrowth
Mitotic rate > 2 per 10 HPF I
If 0 or 1, not helpful for distinction
Ki-67 > 5%
If < 5%, not helpful for distinction
If diagnosis is uncertain, best to diagnose “fibroepithelial lesion” and suggest classification after excision
IMAGE FINDINGS
General Features
Circumscribed mass
May have history of slow or rapid growth
Calcifications not usually present
Mammographic Findings
Circumscribed mass
Partially indistinct margins may be indicative of infiltrative border
MACROSCOPIC FEATURES
General Features
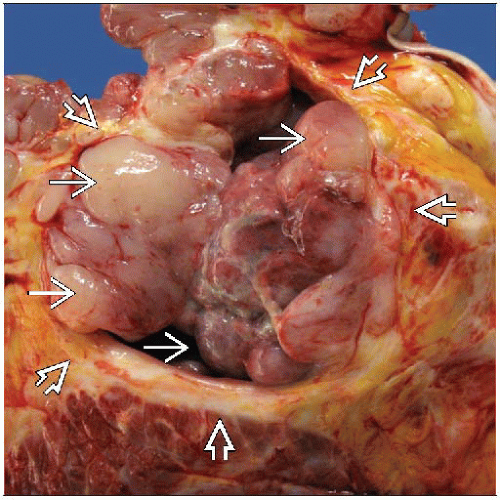
Typically well-defined lobulated masses with bosselated borders
Higher grade PT may show tongues of tumor protruding into adjacent breast parenchyma
May show cleft-like cystic spaces
Size
Most often 4-8 cm (range: 1-40 cm)
Sections to Be Submitted
PT can be very heterogeneous
High-grade features or malignant heterologous elements may be focal
Epithelial component may be focal in high-grade lesions
Tumors should be sampled with at least 1 section per cm of greatest size
Preferable to completely sample PT when possible
Margins should be extensively sampled if undergoing breast-conserving therapy
MICROSCOPIC PATHOLOGY
Histologic Features
Diagnosis of PT requires presence of both spindle stromal cells and benign epithelium
Characteristics of stromal cells are used to distinguish PT from FA and for classification
Cellularity can vary from paucicellular to highly cellular areas
In very cellular areas, cells are organized in parallel arrays (fascicles)
Condensation (increased cellularity) is often found adjacent to epithelium (“cambium” layer)
Stromal overgrowth
Areas of stroma that lack a benign epithelial component (at least 1 HPF)
Does not include paucicellular hyalinized areas
More common in higher grade tumors; uncommon or absent in FAs
Nuclear pleomorphism
Usually mild or moderate
Markedly pleomorphic nuclei are only seen in high-grade PT
Does not include scattered multinucleated cells that may be degenerative in nature
Mitotic rate
Majority of PT will have at least some mitoses
Mitoses in epithelial component are not used for classification
Infiltrative border
Higher grade PT can invade into surrounding breast tissue, creating an irregular border
FA and low-grade PT have pushing circumscribed borders
Must be distinguished from fibroadenomatoid changes in adjacent tissue
Adipose tissue within a PT is usually an indication of invasion; liposarcoma must also be considered
Heterologous elements
Liposarcoma, chondrosarcoma, osteosarcoma, and rhabdomyosarcoma can occur in PT
More common as part of PT than as primary sarcomas
Extensive sampling may be necessary to identify benign epithelium diagnostic of PT
Growth pattern
Intracanicular growth pattern: Stromal cells push and distort epithelium; creates cleft-like spaces lined by epithelium that form leaf-like structures
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree