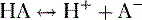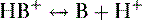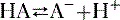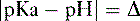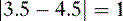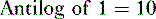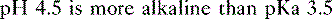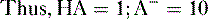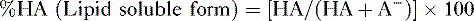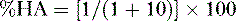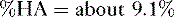Chapter 1 Pharmacokinetics
(4) Lipid to aqueous partition coefficient (PC) determines how readily a drug molecule moves between lipid and aqueous media.
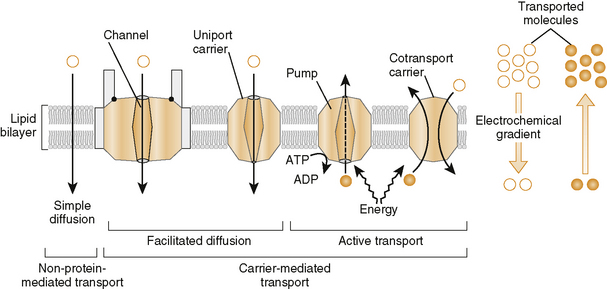
a. Transporters are being identified and characterized that function in movement of molecules into (influx) or out (efflux) of tissues
• See Tables 3-1; and 3-2 of Biochemistry Rapid Review for further details on the movement of molecules and ions across membranes.
b. Numerous transporters such as the ABC (ATP-binding cassette) family including P-glycoprotein or multidrug resistant-associated protein type 1 (MDR1) in the brain, testes, and other tissues play a role in excretion as well as in drug-resistant tumors.
(1) Energy-dependent transporters coupled to ATP hydrolysis (primary active transport); others take place by cotransport proteins (secondary active transport)
a. Drugs in aqueous solutions mix more readily with the aqueous phase at absorptive sites, so they are absorbed more rapidly than those in oily solutions.
b. Drugs in suspension or solid form are dependent on the rate of dissolution before they can mix with the aqueous phase at absorptive sites.
• Drugs in highly concentrated solutions are absorbed more readily than those in dilute concentrations
a. Organs with large surface areas, such as the lungs and intestines, have more rapid drug absorption
Drugs given intramuscularly are absorbed much faster than those given subcutaneously.
Absorbing surface of intestine is much greater than stomach.
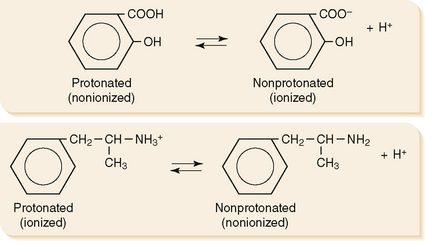
a. For weak acids and weak bases, the pH determines the relative amount of drug in ionized or nonionized form, which in turn affects solubility.
b. Weak organic acids donate a proton to form anions (Fig. 1-3), as shown in the following equation:
where HA = weak acid; H+ = proton; A− = anion
c. Weak organic bases accept a proton to form cations (see Fig. 1-3), as shown in the following equation:
e. The ratio of ionized versus nonionized forms is a function of pKa (measure of drug acidity) and the pH of the environment.
(4) The Henderson-Hasselbalch equation can be used to determine the ratio of the nonionized form to the ionized form.
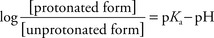
f. Problem: Aspirin is a weak organic acid with a pKa of 3.5. What percentage of aspirin will exist in the lipid soluble form in the duodenum (pH = 4.5)?
Weak organic acids pass through membranes best in acidic environments.
Weak organic bases pass through membranes best in basic environments.
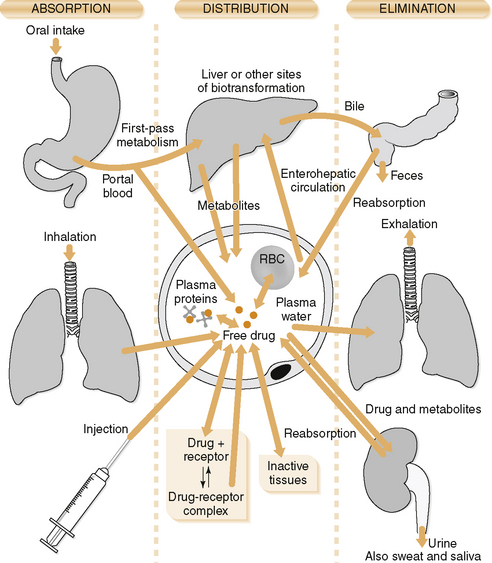
• Bioavailability is the relative amount of the administered drug that reaches the systemic circulation.
• Enzymes in the intestinal flora, intestinal mucosa, and liver metabolize drugs before they reach the general circulation, significantly decreasing systemic bioavailability.
• Bioavailability after oral administration is affected by the extent of disintegration of a particular drug formulation.
1. Two drug formulations with the same bioavailability (extent of absorption) as well as the same rate of absorption are bioequivalent.
TABLE 1-1 Routes of Administration
| Route | Advantages | Disadvantages |
|---|---|---|
| Enteral | ||
| Oral | Destruction of drug by enzymes or low pH (e.g., peptides, proteins, penicillins) Poor absorption of large and charged particles | |
| Rectal | ||
| Sublingual/buccal | Rapid absorption Avoids first-pass metabolism | Absorption of only small amounts (e.g., nitroglycerin) |
| Parenteral | ||
| Intravenous | ||
| Intramuscular | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|