Peritoneal Washings or Lavage in Cancers of the Female Genital Tract
Cytologic sampling of fluid from the peritoneal cavity or, more specifically, from the pelvic cul-de-sac (pouch of Douglas), at the time of surgery was first proposed for ovarian tumors by Keettel and Pixley (1958). The purpose of the procedure was to improve the staging of these tumors. In 1958, Keettel and Pixley published preliminary results indicating that this procedure may provide evidence of spread of ovarian cancer in the absence of visible lesions. In 1986, this concept was incorporated into the official staging of ovarian cancer by the International Federation of Gynecology and Obstetrics (FIGO), shown in Table 15-2.
This staging system attributes an important diagnostic role to the cytologic examination of ascitic and peritoneal fluids: the presence of cancer cells modifies the staging of ovarian tumors from stages Ia or Ib to Ic and from IIa and IIb to IIc. The higher staging calls for a different approach to treatment with the recognition that surgery alone is not likely to be curative of the disease. In current practice the aspiration of pelvic fluid is often supplemented by washings of the cul-de-sac, the fluids being submitted for cytologic examination. Additional data on cytology of ascitic and pleural fluids in ovarian cancer are provided in Chapter 26.
The principal applications of pelvic peritoneal lavage are:
Staging of ovarian, and, somewhat less commonly, other gynecologic cancers
Securing evidence of persisting or recurring cancer during the second-look surgical procedures
Occasional discovery of occult cancer during exploratory laparotomies or laparoscopies for benign disease
Incidental discovery of metastatic cancer from non-gynecologic sites
SECURING AND PROCESSING THE SPECIMEN
McGowen et al (1966) advocated the aspiration of accumulated peritoneal fluid as the first step upon surgical entry into the abdominal cavity, using a laryngeal cannula with a blunted end, attached to a syringe. A washing or lavage of the pelvic peritoneum can be performed using small amounts of normal saline solution or similar fluid that can be repeatedly instilled and reaspirated. If the fluid cannot be processed by the laboratory without delay, the addition of a fixative is recommended (see Chap. 44). Culdocentesis,
an aspiration of pelvic peritoneal fluid across the vaginal wall, may be used for the same purposes. Aspirations may also be performed by skilled operators at the time of a laparoscopy. Luesley et al (1990) advocated the use of direct scrapings or brushings of the peritoneal surfaces as superior to lavage specimens. We do not have any experience with this technique.
an aspiration of pelvic peritoneal fluid across the vaginal wall, may be used for the same purposes. Aspirations may also be performed by skilled operators at the time of a laparoscopy. Luesley et al (1990) advocated the use of direct scrapings or brushings of the peritoneal surfaces as superior to lavage specimens. We do not have any experience with this technique.
The processing of peritoneal aspirates or lavage samples calls for centrifugation of the specimen and preparation of smears or cytospin preparations, as described in Chapter 44. In our experience the use of cell blocks supplementing smears is often diagnostically helpful. Poorly preserved specimens of peritoneal fluid, submitted without fixative after a substantial delay, or unfixed smears prepared by inexperienced personnel may be difficult to interpret. In this type of material, overstained and poorly preserved mesothelial cells may be mistaken for cancer cells. The risk of a falsepositive diagnosis in such cases is substantial.
CYTOLOGY OF PERITONEAL LAVAGE
Benign Cells and Conditions
The principal benign cellular components of peritoneal fluids are mesothelial cells, macrophages, leukocytes, and epithelial cells or cell fragments derived from the peritoneal lining and various benign cysts and other structures. The fluids may also contain “collagen balls,” described by Wojcik and Naylor (1992), calcified debris and, occasionally, psammoma bodies.
Mesothelial Cells
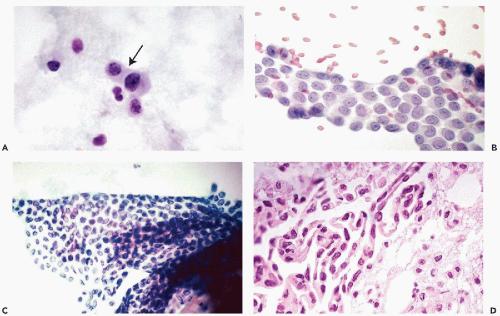
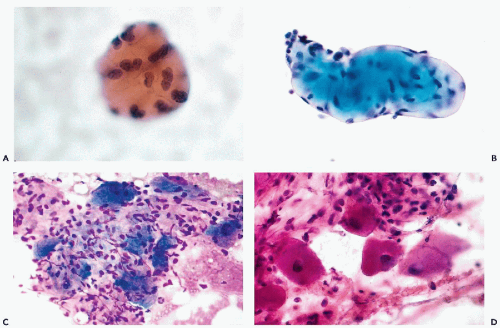
The principal characteristics of mesothelial cells are described in great detail in Chapter 25. In the context of the peritoneal fluid, the mesothelial cells are fairly easy to recognize when well preserved: the cells are of medium size, comparable to small parabasal squamous cells, and have a generally basophilic, delicate cytoplasm, wherein the outer zone is often lighter than the inner, perinuclear zone (Fig. 16-1A). However, depending on the technique used in processing the material and speed of fixation, the mesothelial cells may vary in size and staining properties among specimens. When in flat sheets, the mesothelial cells are often separated from each other by narrow clear gaps or “windows” (Fig. 16-1B,C). Sometimes, long strips of mesothelial cells, forcibly removed from their setting, may be observed in cell blocks (Fig. 16-1D). The cells have central, round, but sometimes slightly indented nuclei, occasionally containing visible small nucleoli (Fig. 16-1A,B). The presence of nucleoli may be troublesome to an inexperienced observer, who may confuse such cells with cancer cells; the fairly monotonous size of the mesothelial cells and their nuclei should prevent the erroneous diagnosis. Through the courtesy of Dr. Wai-Kuen Ng of the Pamela Youde Nethersole Eastern Hospital in Hong Kong, I was privileged to see a very unusual variant of mesothelial cells in peritoneal washings characterized by lobulated nucleus. The term “daisy cells” has been appended to these cells, which were apparently observed before in peritoneal washings. A transition between normal mesothelial cells and “daisy cells” is shown in Figure 16-2. Such cells have not been observed by us in any other fluid and, hence, appear to be a unique feature of peritoneal mesothelial cells.
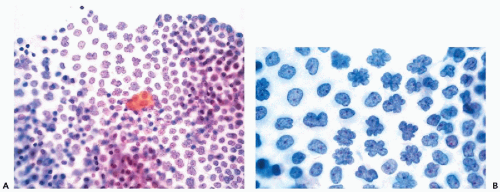
It is not uncommon, however, to see mesothelial cells in large, densely packed sheets wherein the individual cells are difficult to study (Fig. 16-3A,B). As a rule, the diagnosis of cancer should not be made unless the characteristics of cells and their nuclei can be studied in detail; the thick sheets or dense clusters of mesothelial cells are no exception to this rule.
Benign Epithelial Cells
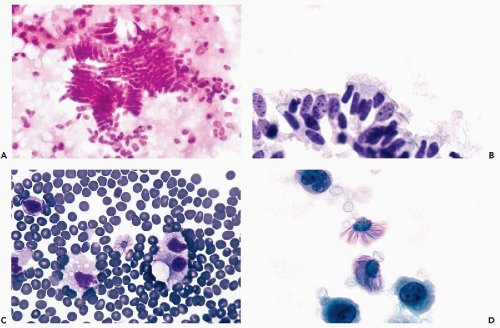
Benign epithelial cells of various types may be observed in peritoneal washings, especially after a vigorous irrigation. These may represent a variety of structures, from ruptured benign inclusion cysts, commonly found on the surfaces of tubes and ovaries, foci of endosalpingiosis and endometriosis, or benign ciliated tubal epithelium. The cells may be of cuboidal or columnar configuration (Figs. 16-3C,D and 16-4A,B) and may appear singly or may form sheets or even papillary clusters, composed of small cuboidal or columnar cells with inconspicuous nuclei and nucleoli. Some of the cells may be ciliated. Detached ciliated tufts of tubal origin may also be observed (Poropatich and Ehya, 1986). Sidawy et al (1987) correlated the presence of the ciliated tufts with stages of menstrual cycle and observed them only in the secretory stage (Fig. 16-4C,D).
Leukocytes and Macrophages
In “first look” specimens and in the absence of cancer, the leukocytes are usually few in number, except in the presence of an inflammatory process. In the latter condition, macrophages accompanied by leukocytes of various types, fibrin and necrotic material may be observed. Cancerous processes may also be accompanied by an inflammatory reaction. In “second look” procedures, a marked inflammatory reaction is often present (see below).
Macrophages may vary in size: most are mononucleated
cells, comparable in size to mesothelial cells, but having finely vacuolated cytoplasm and peripheral, spherical or kidney-shaped nuclei of similar size. Large cytoplasmic vacuoles may occur, pushing the nucleus to the periphery. Evidence of phagocytosis in the form of ingested particles of pigment, such as hemosiderin, helps in the identification of these cells, although sometimes cancer cells are also capable of phagocytosis. Macrophages may also form large, either mono- or multinucleated giant cells; the latter are commonly observed in patients with chronic inflammatory processes or as a reaction to foreign bodies, such as powder, usually observed after a surgical intervention. Macrophages are more common in “second look” procedures.
cells, comparable in size to mesothelial cells, but having finely vacuolated cytoplasm and peripheral, spherical or kidney-shaped nuclei of similar size. Large cytoplasmic vacuoles may occur, pushing the nucleus to the periphery. Evidence of phagocytosis in the form of ingested particles of pigment, such as hemosiderin, helps in the identification of these cells, although sometimes cancer cells are also capable of phagocytosis. Macrophages may also form large, either mono- or multinucleated giant cells; the latter are commonly observed in patients with chronic inflammatory processes or as a reaction to foreign bodies, such as powder, usually observed after a surgical intervention. Macrophages are more common in “second look” procedures.
Collagen Balls
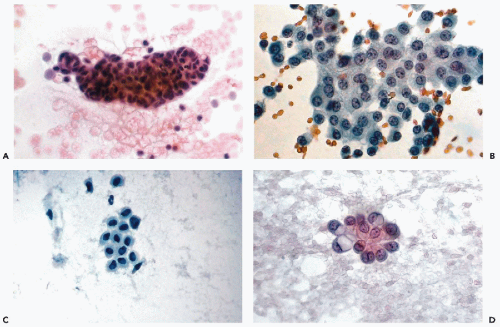
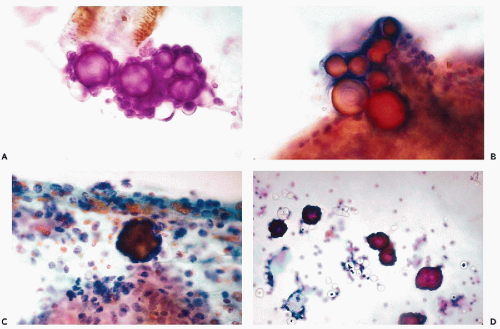
Under this term, Wojcik and Naylor (1992) analyzed the frequency and origin of peculiar homogenous structures, lined by a single layer of cuboidal cells that may be observed in about 5% of pelvic fluids and lavage specimens (Fig. 16-5A,B). In one such case, the structures could be traced to small collagenous excrescences on the surface of an ovary, hence, the conclusion that the small cuboidal cells represent ovarian epithelium. We have observed the collagen balls in benign and malignant peritoneal lavage specimens and, therefore, they have no diagnostic significance.
Foreign, Plant, and Ganglion Cells
During cul-de-sac aspirations performed with a needle-syringe system, a loop of bowel may be inadvertently penetrated. Epithelial cells of intestinal origin or bowel contents in the form of plant cells may be observed. The enteric cells are usually columnar and may occur in large clusters. The plant cells may be identified by a thick, transparent cellulose wall and fine, refractile cytoplasmic granules (see Chapters 8 and 19). Rare findings in aspirates include large ganglion cells, with abundant granular cytoplasm and peripheral nuclei, inadvertently removed from presacral ganglia (Fig. 16-5C,D).
Endometriosis
In abdominal endometriosis two types of cells may be seen side by side: small cuboidal or columnar epithelial cells, usually in small sheets, and, very rarely, very small, spindly stromal cells; these cells are usually accompanied by hemosiderin-laden macrophages. An iron stain may be occasionally helpful in establishing the diagnosis (see Chap. 34). Stowell et al (1997) examined the accuracy of cytologic examination of peritoneal fluids in the diagnosis of endometriosis and concluded that the identification of epithelial cells of endometrial origin is difficult but that the presence
of hemosiderin-laden macrophages should alert the pathologist to the possibility of this disorder.
of hemosiderin-laden macrophages should alert the pathologist to the possibility of this disorder.
Endosalpingiosis
In endosalpingiosis the fluids are characterized by the presence of calcified debris and psammoma bodies that can be numerous, and are sometimes surrounded by inconspicuous, small, benign epithelial cells (Fig. 16-6; see also Fig. 15-7). Psammoma bodies may also occur within fragments of glandular structures or surrounded by inflammatory exudate and cell debris (Fig. 16-6C).
In incidental biopsies, small cystic structures, lined by cuboidal, occasionally ciliated, epithelial cells and containing calcified debris or psammoma bodies, may be observed on the surface of the fallopian tubes, the ovaries, and elsewhere in the peritoneum (see Fig. 15-7). For a discussion on the possible relationship of endosalpingiosis to psammocarcinoma, see Chapter 15.
The presence of psammoma bodies in peritoneal lavage may be perplexing, particularly in the presence of ovarian abnormalities that may be unrelated to the cytologic findings (Sidawy and Silverberg, 1987). In general, psammoma bodies or calcified debris observed in peritoneal washings, do not have the same diagnostic significance as in vaginal and cervical material (see Chap. 15) or in effusions (see Chap. 26) and, unless accompanied by cancer cells, should be interpreted with great caution. Focal calcium deposits, not uncommon in the peritoneum, may be dislodged by vigorous lavage and may mimic psammoma bodies.
Diagnostic Problems in the Absence of Cancer