Peripheral Arterial Disease
KEY CONCEPTS
![]() The prevalence of peripheral arterial disease is dependent on age and the presence of traditional risk factors for cardiovascular disease and many patients are undiagnosed; undiagnosed patients have substantial risk for coronary and cerebrovascular events.
The prevalence of peripheral arterial disease is dependent on age and the presence of traditional risk factors for cardiovascular disease and many patients are undiagnosed; undiagnosed patients have substantial risk for coronary and cerebrovascular events.
![]() The clinical presentation of peripheral arterial disease is variable and includes a range of symptoms. The two most common characteristics of peripheral arterial disease are intermittent claudication and pain at rest in the lower extremities.
The clinical presentation of peripheral arterial disease is variable and includes a range of symptoms. The two most common characteristics of peripheral arterial disease are intermittent claudication and pain at rest in the lower extremities.
![]() The ankle-brachial index (ABI) is a simple, noninvasive, quantitative test that has been proven to be a highly sensitive and specific tool in the diagnosis of peripheral arterial disease.
The ankle-brachial index (ABI) is a simple, noninvasive, quantitative test that has been proven to be a highly sensitive and specific tool in the diagnosis of peripheral arterial disease.
![]() As with any atherosclerotic condition, several risk factors play an important role in the morbidity and mortality of peripheral vascular disease. Many of these risk factors are modifiable with the help of various nonpharmacologic and pharmacologic interventions.
As with any atherosclerotic condition, several risk factors play an important role in the morbidity and mortality of peripheral vascular disease. Many of these risk factors are modifiable with the help of various nonpharmacologic and pharmacologic interventions.
![]() Nonpharmacologic interventions such as smoking cessation and walking exercise programs have the ability to positively impact several of the pathophysiologic abnormalities present in patients with peripheral arterial disease.
Nonpharmacologic interventions such as smoking cessation and walking exercise programs have the ability to positively impact several of the pathophysiologic abnormalities present in patients with peripheral arterial disease.
![]() Data proving that antiplatelet therapies can prevent or delay the progression of peripheral arterial disease are currently unavailable. However, aspirin therapy has repeatedly been proven to significantly reduce serious vascular events in these “high-risk” patients and, in the absence of contraindications, is highly recommended.
Data proving that antiplatelet therapies can prevent or delay the progression of peripheral arterial disease are currently unavailable. However, aspirin therapy has repeatedly been proven to significantly reduce serious vascular events in these “high-risk” patients and, in the absence of contraindications, is highly recommended.
![]() After appropriate exercise therapy and therapeutic lifestyle changes have been implemented, patients who continue to experience severe intermittent claudication may benefit from additional pharmacologic therapy with cilostazol.
After appropriate exercise therapy and therapeutic lifestyle changes have been implemented, patients who continue to experience severe intermittent claudication may benefit from additional pharmacologic therapy with cilostazol.
Peripheral arterial disease (PAD), the most common form of peripheral vascular disease, is a manifestation of progressive narrowing of arteries due to atherosclerosis.1 PAD is associated with elevated risk of cardiovascular disease (CVD) morbidity and mortality, even in the absence of prior history of acute myocardial infarction (AMI), stroke, or other manifestations of CVD.2 Patients with PAD have approximately the same relative risk of death from CVD as do patients with a history of coronary or cerebrovascular disease, and PAD should be considered a surrogate marker of subclinical coronary artery disease (CAD) and other vascular territories.1 The treatment of PAD focuses on decreasing the functional impairment caused by symptoms of intermittent claudication (IC) through nonpharmacologic and pharmacologic therapy and by minimizing the impact of other cardiovascular risk factors.1
EPIDEMIOLOGY
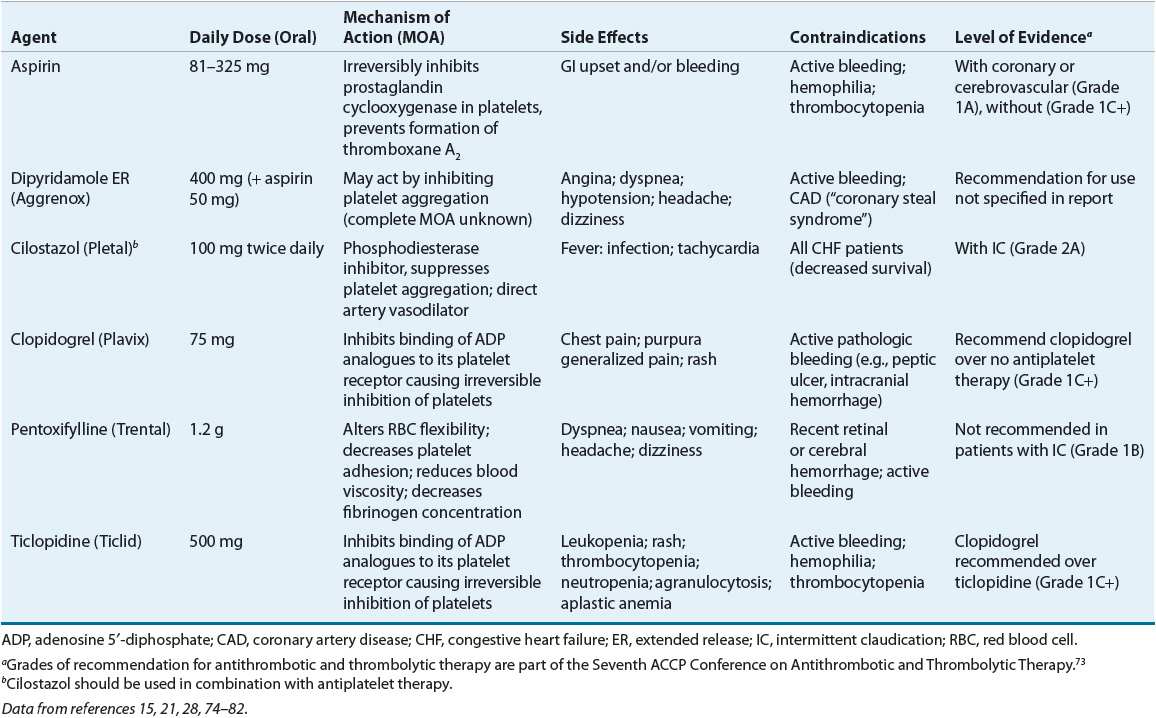
![]() PAD should be defined with the ankle-brachial index (ABI) and compatible signs and symptoms. The normal range is 1 to 1.40 and ABI ≤0.90 in either leg or >1.40 representing noncompressible arteries. The National Health and Nutrition Examination Survey (NHANES) found a 4.6% prevalence of PAD among adults aged 40 years and older in the United States.1 The prevalence of PAD is highly dependent on age, being infrequent in younger individuals and common in older individuals (Fig. 12-1). In age- and gender-adjusted logistic regression analyses, black race/ethnicity (odds ratio [OR] 2.83), current smoking (OR 4.46), diabetes (OR 2.71), hypertension (HTN; OR 1.75), hypercholesterolemia (OR 1.68), and impaired renal function (estimated glomerular filtration rate less than 60 mL/min/1.73 m2) (OR 2) were associated with more prevalent PAD.1,6 Individuals with PAD are also more likely to have a self-reported history of any CAD or CVD but, interestingly, no association with elevated body mass index. The reported relative risk of death from CVD in patients with PAD is reported to range from 2 to 5.1 in patients with or without CVD and 2.9 to 5.7 in patients with known CVD.3 CVD accounts for 75% of all deaths in patients with PAD.4 The risk of death is approximately the same in men and women and is elevated even in asymptomatic patients. Annual mortality is 25% in patients with critical leg ischemia who have the lowest ABI values.5
PAD should be defined with the ankle-brachial index (ABI) and compatible signs and symptoms. The normal range is 1 to 1.40 and ABI ≤0.90 in either leg or >1.40 representing noncompressible arteries. The National Health and Nutrition Examination Survey (NHANES) found a 4.6% prevalence of PAD among adults aged 40 years and older in the United States.1 The prevalence of PAD is highly dependent on age, being infrequent in younger individuals and common in older individuals (Fig. 12-1). In age- and gender-adjusted logistic regression analyses, black race/ethnicity (odds ratio [OR] 2.83), current smoking (OR 4.46), diabetes (OR 2.71), hypertension (HTN; OR 1.75), hypercholesterolemia (OR 1.68), and impaired renal function (estimated glomerular filtration rate less than 60 mL/min/1.73 m2) (OR 2) were associated with more prevalent PAD.1,6 Individuals with PAD are also more likely to have a self-reported history of any CAD or CVD but, interestingly, no association with elevated body mass index. The reported relative risk of death from CVD in patients with PAD is reported to range from 2 to 5.1 in patients with or without CVD and 2.9 to 5.7 in patients with known CVD.3 CVD accounts for 75% of all deaths in patients with PAD.4 The risk of death is approximately the same in men and women and is elevated even in asymptomatic patients. Annual mortality is 25% in patients with critical leg ischemia who have the lowest ABI values.5
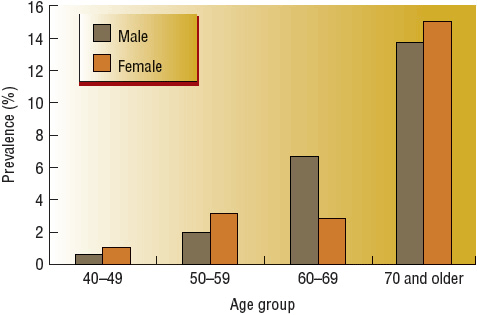
FIGURE 12-1 Prevalence of peripheral arterial disease by age and gender.
More than ∼8.5 million (estimated range 4 to 7 million) adults aged 40 years have PAD. Ninety-five percent of individuals with PAD have at least one cardiovascular risk factor; the majority of patients have multiple risk factors for CVD.6 Based on the PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) program, the prevalence of PAD in primary care practices is high, yet physician awareness of the PAD diagnosis is relatively low.7 In this cross-sectional study, PAD was detected in 29% of 6,979 patients. Eighty-three percent of the patients were aware of their diagnosis, but only 49% of their patients’ physicians were aware. The reason for this observation is that patient self-report of symptoms and the use of questionnaires to detect PAD are not sufficiently sensitive and specific to reproducibly diagnose PAD and the cardinal symptom of PAD—IC—is present in the minority of patients (1% to 27%, typically ∼10%).1,3,8 A simple ABI measurement will identify a large number of patients with previously unrecognized PAD. Atherosclerosis risk factors were very prevalent in PAD patients, but these patients received less intensive treatment for lipid disorders and HTN and were prescribed antiplatelet therapy less frequently than were patients with CVD. These results demonstrate that underdiagnosis of PAD in primary care practice may be a barrier to effective secondary prevention of the high ischemic cardiovascular risk associated with PAD.7 Because of the systemic nature of atherosclerosis and the high risk of ischemic events, patients with PAD should be considered for secondary prevention strategies including aggressive risk factor modification and antiplatelet drug therapy.3,8
ETIOLOGY AND PATHOPHYSIOLOGY
PAD is most commonly a manifestation of systemic atherosclerosis in which the arterial lumen of the lower extremities becomes progressively occluded by atherosclerotic plaque.4,8 The major risk factors for the development of atherosclerosis are older age (greater than 40 years), cigarette smoking, diabetes mellitus, hypercholesterolemia, HTN, and hyperhomocysteinemia.3,4,9 The arteries most commonly involved, in order of occurrence, are the femoropopliteal-tibial, aortoiliac, carotid and vertebral, splenic and renal, and brachiocephalic.10 Familial hypercholesterolemia (FH) leading to hypercholesterolemia and elevated low-density lipoprotein (LDL) levels are associated with accelerated development of atherosclerosis earlier and with more severe symptoms (e.g., IC) and abnormal blood flow studies compared with controls.10,11 Intima–media thickness can be used as a surrogate phenotype for cardiovascular risk in FH and carotid and/or femoral artery atherosclerosis results in increased intima–media thickness and it is correlated to cardiovascular risk in FH patients compared with normolipidemic individuals.
CLINICAL PRESENTATION AND DIAGNOSIS
![]() The clinical presentation of PAD is variable, ranging from no symptoms at all (typically early in the disease) to pain and discomfort (Table 12-1). This finding was illustrated in a study by Wang et al.,12 who attempted to aid the diagnosis of PAD by using defined categories of exertional leg pain in patients with and without PAD. They determined that not one of the five categories of leg pain (no pain, pain on exertion and rest, noncalf pain, atypical calf pain, and classic claudication) was sufficiently sensitive or specific to enable a link to a PAD diagnosis. The two most common characteristics of PAD are IC and pain at rest in the lower extremities.11,12,25 IC is generally regarded as the primary indicator of PAD. It is described as reproducible fatigue, discomfort, cramping, pain, or numbness in the affected extremities (typically the buttock, thigh, or calf) during exercise and is resolved within a few minutes with rest.11,13–15,25,26 Symptoms of IC occur during exercise as the increase in blood flow is limited by occlusive atherosclerotic lesions in the peripheral arteries leading to an inability for oxygen supply to meet the demands of increased metabolic demand by the muscles.26 Resting pain typically occurs later in the disease when the blood supply is not adequate to perfuse the extremity (critical limb ischemia). This most often can be felt at night in the feet (typically the toes or heel) while the patient is lying in bed.11,12,25 Although IC is the primary indicator of PAD, it alone cannot be used to diagnose PAD. Unfortunately only –10% of patients present with classical IC.8 As explained by the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TransAtlantic Inter-Society Consensus [TASC] II),26 patients with PAD may not have symptoms of IC because they may have a sedentary lifestyle or some other condition that may be limiting the ability to exercise.
The clinical presentation of PAD is variable, ranging from no symptoms at all (typically early in the disease) to pain and discomfort (Table 12-1). This finding was illustrated in a study by Wang et al.,12 who attempted to aid the diagnosis of PAD by using defined categories of exertional leg pain in patients with and without PAD. They determined that not one of the five categories of leg pain (no pain, pain on exertion and rest, noncalf pain, atypical calf pain, and classic claudication) was sufficiently sensitive or specific to enable a link to a PAD diagnosis. The two most common characteristics of PAD are IC and pain at rest in the lower extremities.11,12,25 IC is generally regarded as the primary indicator of PAD. It is described as reproducible fatigue, discomfort, cramping, pain, or numbness in the affected extremities (typically the buttock, thigh, or calf) during exercise and is resolved within a few minutes with rest.11,13–15,25,26 Symptoms of IC occur during exercise as the increase in blood flow is limited by occlusive atherosclerotic lesions in the peripheral arteries leading to an inability for oxygen supply to meet the demands of increased metabolic demand by the muscles.26 Resting pain typically occurs later in the disease when the blood supply is not adequate to perfuse the extremity (critical limb ischemia). This most often can be felt at night in the feet (typically the toes or heel) while the patient is lying in bed.11,12,25 Although IC is the primary indicator of PAD, it alone cannot be used to diagnose PAD. Unfortunately only –10% of patients present with classical IC.8 As explained by the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TransAtlantic Inter-Society Consensus [TASC] II),26 patients with PAD may not have symptoms of IC because they may have a sedentary lifestyle or some other condition that may be limiting the ability to exercise.
TABLE 12-1 Clinical Presentation
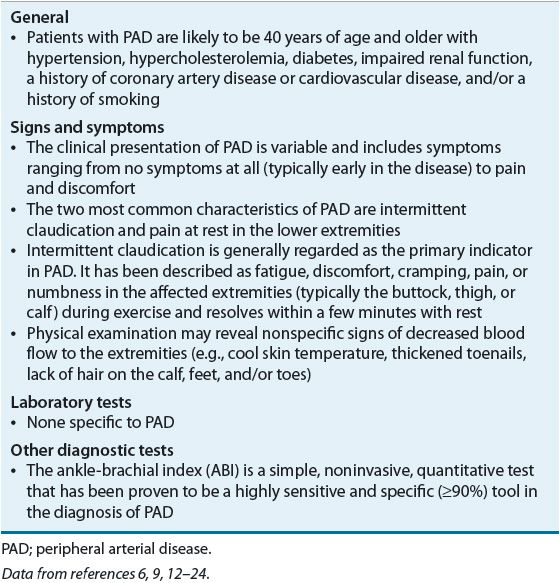
As with any good medical encounter, a detailed patient history of symptoms and atherosclerosis risk factors (e.g., smoking, HTN, hyperlipidemia, and diabetes) can be helpful in the diagnosis of PAD. Unfortunately, as illustrated by the PARTNERS program, providers who rely on a history alone will miss approximately 85% to 90% of patients with PAD.16 Therefore, examination of the patient is vital to proper diagnosis. Requesting that the patient remove socks and shoes may reveal nonspecific signs of decreased blood flow to the extremities (e.g., cool skin temperature, shiny skin, thickened toenails, lack of hair on the calf, feet, and/or toes) or, in severe cases, visible sores or ulcers that are slow to heal and may even be black in appearance.11,12,16,17,25
An important criterion for the accurate diagnosis of PAD is the exclusion of other conditions that possess similar signs and symptoms. Differential diagnosis should rule out other neurologic conditions (e.g., peripheral neuropathy), inflammatory conditions (e.g., arthritis), and vascular conditions (e.g., deep venous thrombosis or venous congestion) that may mimic PAD.11,15,18
![]() The ABI is a simple, noninvasive, quantitative test that has been proven to be a highly sensitive and specific (≥90%) tool in the diagnosis of PAD.13,19,27 For measurement of the ABI, the patient lies in the supine position as the systolic blood pressure is measured at the brachial arteries on both arms and the dorsalis pedis and posterior tibial arteries of the legs with a standard sphygmomanometer and a continuous-wave Doppler device. The pressures obtained at the dorsalis pedis and posterior tibial arteries are averaged and divided by the mean measurement taken at the left and right brachial arteries.3,16,20,26,28 An ABI of 1 to 1.40 is considered normal while a measurement under 0.9 is consistent with PAD. ABI from 0.7 to 0.9 correlates with mild PAD, 0.4 to 0.7 indicates moderate disease, and under 0.4 denotes severe PAD.4,14,20 An ABI of >1.40 is consistent with noncompressible arteries. In addition to providing diagnostic information, the ABI measurement has been shown to be a strong predictor of future cardiovascular events associated with PAD.21,29 The ABI can also be useful after a test of exercise tolerance (e.g., 5 minutes on a treadmill or 30 to 50 repetitions of heel raises). Patients with PAD will demonstrate a significant drop in the ABI after exercise, but their pain will be normal or unchanged. ABI can rule out PAD and suggest alternate diagnoses.4,14,16,26 ABI can be considered as a useful tool in diagnosing both symptomatic and nonsymptomatic patients at high risk of PAD.7,30
The ABI is a simple, noninvasive, quantitative test that has been proven to be a highly sensitive and specific (≥90%) tool in the diagnosis of PAD.13,19,27 For measurement of the ABI, the patient lies in the supine position as the systolic blood pressure is measured at the brachial arteries on both arms and the dorsalis pedis and posterior tibial arteries of the legs with a standard sphygmomanometer and a continuous-wave Doppler device. The pressures obtained at the dorsalis pedis and posterior tibial arteries are averaged and divided by the mean measurement taken at the left and right brachial arteries.3,16,20,26,28 An ABI of 1 to 1.40 is considered normal while a measurement under 0.9 is consistent with PAD. ABI from 0.7 to 0.9 correlates with mild PAD, 0.4 to 0.7 indicates moderate disease, and under 0.4 denotes severe PAD.4,14,20 An ABI of >1.40 is consistent with noncompressible arteries. In addition to providing diagnostic information, the ABI measurement has been shown to be a strong predictor of future cardiovascular events associated with PAD.21,29 The ABI can also be useful after a test of exercise tolerance (e.g., 5 minutes on a treadmill or 30 to 50 repetitions of heel raises). Patients with PAD will demonstrate a significant drop in the ABI after exercise, but their pain will be normal or unchanged. ABI can rule out PAD and suggest alternate diagnoses.4,14,16,26 ABI can be considered as a useful tool in diagnosing both symptomatic and nonsymptomatic patients at high risk of PAD.7,30
Other noninvasive tools are available for the diagnosis of PAD. One study has suggested a calculation that takes into consideration the patient’s history of AMI and the number of auscultated and palpated posterior tibial arteries.31,32 Magnetic resonance angiography (MRA) can be used to examine the presence and location of significant stenosis, or lack thereof, and is a reasonable option in patients who are being considered for surgical revascularization.33 Similarly, computed tomographic angiography (CTA) can be used to determine the presence of significant stenosis and soft-tissue diagnostic information that may be associated with PAD (e.g., aneurysms).33,34 However, as ABI is a sufficient means of diagnosis, arteriography is not necessary or encouraged.15,25,27
TREATMENT
Peripheral Arterial Disease
Goals of Treatment
PAD is the result of atherosclerotic plaque formation in the arteries that results in decreased blood flow to the legs. Several of the treatment goals for these patients involve the reduction of confounding variables that attribute to the disease process, progress, and eventual outcome. Specific goals should include increasing maximal walking distance, duration, and pain-free walking, improving control of comorbid conditions contributing to the morbidity of the condition (e.g., HTN, hyperlipidemia, and diabetes), improvement in overall quality of life, and reduction in cardiovascular complications and death.
General Approach to Treatment
![]() As with any atherosclerotic condition, several risk factors play an important role in the morbidity and mortality of PAD. Many of these risk factors are modifiable with the help of various nonpharmacologic and pharmacologic interventions.
As with any atherosclerotic condition, several risk factors play an important role in the morbidity and mortality of PAD. Many of these risk factors are modifiable with the help of various nonpharmacologic and pharmacologic interventions.
Nonpharmacologic Therapy
Smoking Cessation
![]() Not only cigarette smoking increases the risk of developing PAD and other cardiovascular disorders, but also the duration and quantity smoked can negatively impact disease progression (e.g., increase the risk of amputation) and increase mortality.3,18,21,26,29,35–38 As a result, providers must advise patients to quit and should offer nonpharmacologic and pharmacologic means to aid the patient in that goal. Individual or group behavior modification therapy with or without the addition of certain antidepressants (e.g., bupropion), varenicline, or nicotine replacement therapy (e.g., gum or patches) has been proven effective in numerous studies. Varenicline has demonstrated superior quit rates compared with nicotine replacement therapy and bupropion.8 Other forms of tobacco use should be discouraged as well. Reassessment of smoking status and progress encouragement at each encounter can help to reemphasize to the patient the vital importance of this lifestyle change.
Not only cigarette smoking increases the risk of developing PAD and other cardiovascular disorders, but also the duration and quantity smoked can negatively impact disease progression (e.g., increase the risk of amputation) and increase mortality.3,18,21,26,29,35–38 As a result, providers must advise patients to quit and should offer nonpharmacologic and pharmacologic means to aid the patient in that goal. Individual or group behavior modification therapy with or without the addition of certain antidepressants (e.g., bupropion), varenicline, or nicotine replacement therapy (e.g., gum or patches) has been proven effective in numerous studies. Varenicline has demonstrated superior quit rates compared with nicotine replacement therapy and bupropion.8 Other forms of tobacco use should be discouraged as well. Reassessment of smoking status and progress encouragement at each encounter can help to reemphasize to the patient the vital importance of this lifestyle change.
Exercise
![]() Walking exercise programs for patients with PAD have been proven to result in an increase in walking duration and distance, an increase in pain-free walking, and a delayed onset of claudication by 179%.9,17,18,35,36,39–48 Walking, or any aerobic exercise program conducted under the supervision of a healthcare provider, has the ability to positively impact several of the pathophysiologic abnormalities present in patients with PAD. Benefits of exercise programs include improving diabetes and lipid management, reducing weight, improving blood viscosity and flow, and reducing blood pressure.49 Walking distance can also be used as a prognostic tool for future outcomes in patients with normal and impaired ABIs. A recent study by de Liefde et al.50 examined patients with normal ABI (≥0.90) and impaired ABI (<0.90) in relation to walking distance. It was demonstrated that walking impairment in conjunction with impaired ABI was associated with higher cardiovascular events, including death. Other studies have likewise observed a link between impaired exercise/walking distance and negative long-term outcomes in patients with PAD.51,52
Walking exercise programs for patients with PAD have been proven to result in an increase in walking duration and distance, an increase in pain-free walking, and a delayed onset of claudication by 179%.9,17,18,35,36,39–48 Walking, or any aerobic exercise program conducted under the supervision of a healthcare provider, has the ability to positively impact several of the pathophysiologic abnormalities present in patients with PAD. Benefits of exercise programs include improving diabetes and lipid management, reducing weight, improving blood viscosity and flow, and reducing blood pressure.49 Walking distance can also be used as a prognostic tool for future outcomes in patients with normal and impaired ABIs. A recent study by de Liefde et al.50 examined patients with normal ABI (≥0.90) and impaired ABI (<0.90) in relation to walking distance. It was demonstrated that walking impairment in conjunction with impaired ABI was associated with higher cardiovascular events, including death. Other studies have likewise observed a link between impaired exercise/walking distance and negative long-term outcomes in patients with PAD.51,52
The American College of Cardiology/American Heart Association (ACC/AHA) Guidelines for the Management of PAD recommend supervised exercise training for patients with IC, for a minimum of 30 to 45 minutes, to be performed at least three times per week for a minimum of 12 weeks.33 During exercise sessions, walking should be performed at a speed and grade of incline to produce the symptoms of IC within 3 to 5 minutes. The patient should stop walking when the symptoms become moderate in intensity, wait for the symptoms to resolve, and then resume walking, thus repeating the cycle for the duration of the session.26 A prospective, observational study performed by Gardner et al. concluded that PAD patients with higher physical activity (as measured with a vertical accelerometer) have reduced mortality and cardiovascular events compared with those with low physical activity, regardless of confounders.45 Exercise treadmill walking testing should be repeated at regular intervals (e.g., quarterly to biannually) to assess improvement or decline in walking duration and distance, as well as the time to pain onset while performing this activity. The type of aerobic activity recommended, as well as the duration and frequency of the activity, should be individually designed on a patient-to-patient basis.
Surgical Interventions
Various surgical procedures are available for patients with severe, debilitating claudication who have attempted, and failed, other means of nonpharmacologic and pharmacologic therapy. The TASC document on PAD provides clear recommendations for invasive therapy.27 First, there must be a lack of adequate response to exercise therapy and risk factor modification. Second, the patient must have severe disability from IC resulting in impairment of daily activities. Third, there must be a thorough evaluation of the risks versus benefits of an invasive intervention including probability of success, the anticipated future course of the disease if an intervention is not performed, as well as an evaluation of concomitant disease states.27 The decision to attempt percutaneous revascularization is often made with the guidance of diagnostic angiography. Angiography can help to identify the location and size of lesions and provide valuable information as to the likelihood of success with surgical revascularization.27
Percutaneous transluminal angioplasty (PTA) is an example of an invasive treatment for PAD. A randomized controlled clinical trial performed by Whyman et al.53 determined that in a 2-year post intervention, PTA outcomes on maximum walking distance and ABI were not significantly different than in patients who had only received daily low-dose aspirin (acetylsalicylic acid [ASA]; P >0.05). Nevertheless, patients who had received PTA had significantly fewer occluded arteries (P = 0.003), but the true clinical significance of this finding was not able to be realized in the time allotted for the study. PTA typically is reserved for patients whose lifestyle and/or job performance are compromised secondary to claudication despite adequate pharmacologic interventions and exercise.9,37 Another consideration for balloon angioplasty is life expectancy. PTA is preferred over surgery if life expectancy is <2 years.8
Stent placement in PAD patients has also been an area of study and controversy. A meta-analysis examining the use of stent placement versus PTA for the treatment of aortoiliac occlusive disease determined that, although stent placement and PTA yielded similar complication and mortality rates, posttreatment ABI was more improved with stents (0.87 with PTA and 0.76 with stents, P <0.03) and the risk of long-term failure was 39% less with stent placement.54 However, other studies have not demonstrated improvement in patency rates in peripheral arteries versus PTA alone.13,55 The TASC document provides specific recommendations for PTA, with or without stenting, depending on how diffuse the disease process is, the number and size of the lesions, and the location of the lesions.27
For patients with severe IC resulting in critical leg ischemia, physicians may need to discuss alternate surgical interventions including aortofemoral bypass, femoral popliteal bypass, or even amputation.13,14,39
Pharmacologic Therapy
Hypertension
HTN is a major risk factor for PAD and can lead to AMI, stroke, heart failure (HF), and death.56 Current guidelines recommend the treatment goal for blood pressure in patients with PAD to mirror those in patients with documented CVD, 130/85 mm Hg.38,56 Although the Heart Outcomes Prevention Evaluation (HOPE)57 study demonstrated that angiotensin-converting enzyme (ACE) inhibitors reduced not only blood pressure but also other cardiovascular events (e.g., AMI, stroke, and death) in high-risk patients, including those with PAD, no specific class of antihypertensives is recommended over another for the treatment of HTN in patients with PAD. Therefore, selection of drug therapy for HTN should be made in accordance with the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII)56 on the basis of comorbid disease states, drug costs and availability, drug allergies, or other possible limiting factors. For example, patients with concomitant Raynaud’s phenomenon may benefit from calcium channel blockers while patients with documented CAD may receive a dual benefit by the selection of a β-blocker.38,56,58 Hesitation to use β-blockers in patients with PAD without harm was recently supported with the publication of a review of six randomized controlled trials by Paravastu et al.59 The review concluded that there was no evidence of harm in the use of these agents in patients with PAD; however, β-blockers should be used with caution in patients with critical leg ischemia where acute lowering of blood pressure is contraindicated.59 In 2012, Tseng et al. described a relationship between ACE insertion/deletion polymorphisms with the DD genotype carrying greatest risk for cardiovascular outcomes.60 It is not known if ACE inhibitors would lead to greater risk reduction. Dosing, monitoring guidelines, and contraindications for specific agents used in the treatment of HTN may be found in Chapter 3.61
Hyperlipidemia
Although it has been shown that a reduction in lipid levels can reduce the progression of PAD and the severity of claudication, the current recommendations for the management of hyperlipidemia in PAD are based on only a few small studies and sub-hoc analyses from larger trials.3,38,62–64 The Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP III) considers PAD to be in the category of highest risk, or a coronary heart disease (CHD) risk equivalent. Therefore, it was recommended by the Expert Panel that levels of LDL be maintained at <100 mg/dL (<2.59 mmol/L) and non–high-density lipoprotein (non-HDL) levels (total cholesterol – HDL cholesterol) at <130 mg/dL (<3.36 mmol/L).62 Results of clinical trials conducted since the time of this recommendation, specifically the Heart Protection Study (HPS)64 and the Pravastatin or Atorvastatin Evaluation and Infection—Thrombolysis in Myocardial Infarction (PROVE IT)65 trial, have led many clinical experts to now recommend an LDL goal of <70 mg/dL (<1.81 mmol/L) for additional retardation of atherosclerotic plaque formation in persons considered to be at very high risk, including patients with PAD.13 Regardless of the goal LDL chosen, initiation of patient therapeutic lifestyle changes (TLC; e.g., reduction in saturated fat, weight reduction, and increased physical activity) is vital to achieving these recommendations.10,62 Unfortunately, in many cases, TLC alone will not achieve the desired goals.
Several options are available for the initiation of drug therapy for LDL lowering in patients with PAD. Statins, bile acid sequestrants, and nicotinic acid are all effective treatment options. However, in most cases, statins are the preferred starting agent in this patient population.16,39,62,64 As proven in the HPS, simvastatin not only demonstrated potent action in reducing LDL but also provided a significant reduction in cardiovascular events overall (e.g., AMI, stroke, and death).64 If an increase in HDL levels is also necessary, niacin should be considered alone or in combination with a statin without the fear of worsening glucose metabolism, as previously believed.3,14,28,58,66 Niacin has not been shown to be effective in increasing exercise time when compared with dietary intervention.67 Dosing, monitoring guidelines, and contraindications for specific agents may be found in Chapter 11.
Diabetes Mellitus
A meta-analysis of over 95,000 diabetic patients provided additional support for the accepted premise that glycemic control serves as a risk factor for CVD.68 The analysis demonstrated an increasing risk of death from cardiovascular events as blood glucose concentrations increased, with the same relationship observed even at levels below the threshold of clinically defined diabetes mellitus. This relationship is just one illustration of the criticality of good glycemic control. Due to the high prevalence of PAD among diabetic patients, the American Diabetes Association recommends ABI screening for PAD in all diabetics older than 50 years.69 Due to the presence of peripheral neuropathy, patients with diabetes may be less likely to experience or report symptoms of PAD and the first sign may be as drastic as the appearance of a gangrenous foot ulcer. Therefore, although there is currently a lack of randomized controlled studies illustrating that the degree of glycemic control is predictive of the extent of PAD present, it is widely recommended that all patients with concomitant diabetes and PAD maintain good glycemic control, as evidenced by a hemoglobin A-1c level of <7% (<0.07; <53 mmol/mol Hb).3,27,28,38,40,69,70 This recommendation is supported by a prospective cohort study of 1,894 diabetic patients, which demonstrated that patients with poor glucose control (A-1c >7.5% [>0.075; >58 mmol/mol Hb]) were five times more likely to develop IC and also to be hospitalized for PAD compared with those with a hemoglobin A-1c <6% (<0.06; <42 mmol/mol Hb).71 Despite this, a study by Rehring et al.72 of 365 patients with known PAD and concomitant diabetes showed that only 45.8% of these patients had a hemoglobin A-1c <7% (<0.07; <53 mmol/mol Hb). Oral antidiabetic agents, insulin regimens, as well as other pharmacologic and nonpharmacologic strategies to reduce the risk of complications associated with diabetes mellitus are discussed at length in Chapter 57.
Antiplatelet Drug Therapy
See Table 12-2.
TABLE 12-2 Pharmacotherapy Options for Patients with Peripheral Arterial Disease