Perioperative Management of Morbid Obesity
Daniel B. Jones
Stephanie B. Jones
C Morbid Obesity
More and more Americans are overweight, one-third of the population is obese, and the disease is fast becoming the leading cause of preventable death. Obesity is best defined by body mass index (BMI) and can be quickly calculated from a table or online by measuring weight (kg) and height (m). Overweight is defined as BMI greater than 25 kg/m2, while BMI greater than 30 kg/m2 signifies obesity. While prevention is the key to battling the national epidemic, diet and exercise have proven unsuccessful at reversing weight gain long term. The National Institutes of Health (NIH) has recognized that bariatric surgery can achieve durable weight loss in the morbidly obese patient. In general, weight loss surgery is considered medically necessary with a BMI of 40 kg/m2 or BMI of 35 kg/m2 with other weight-related medical illnesses. Today nearly 200,000 weight loss operations are performed annually in the United States. In an era of patient safety and best practices, lessons learned caring for the bariatric surgery patient can now be applied to the obese patient undergoing other general surgery operations.
Obesity is associated with many comorbid conditions, including hypertension, obstructive sleep apnea (OSA), type 2 diabetes, lower back pain, and headache (Table 1). Obesity is also implicated in increased rates of colon, breast, and other cancers. It is no surprise that heavier patients don’t live as long as healthier, thinner patients. Fortunately, several studies have clearly proven than weight loss reverses many of these conditions and patients live longer after weight loss. In fact, these outcome studies are the basis of economic studies that demonstrate weight loss surgery to be very cost-effective to society and a viable treatment strategy.
The field of bariatric surgery has experienced exponential growth to keep pace with the epidemic of obesity in the United States. With the high demand for weight loss surgery, surgeons with varied backgrounds and training gravitated to the specialty. Complications let to significant scrutiny, which in turn led to evolution of best practice recommendations, accreditation requirements, benchmarks, and outcome measures.
Accreditation standards were established by the American Society for Metabolic and Bariatric Surgery (ASMBS) and the American College of Surgeons (ACS). Requirements are listed for surgeon training, annual operative volume, and continuing education. Outcomes are tracked and reported to national databases (NSQIP, BOLD) and surgeon outcomes can be compared with national benchmarks. Accreditation is designed to ensure proper facilities (beds, lifts, computed tomography (CT) scanners, intensive care units), multidisciplinary teams, and personnel for optimal care. The review further reinforces the need for an environment sensitive to the physical and psychosocial needs of the obese patient and their family members.
Assessment for Weight Loss Surgery
The most commonly performed operations for weight loss include the Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy (Fig. 1). While subsequent chapters will describe the techniques, benefits, and complications, the preoperative evaluation specific for weight loss surgery is worthy of mention. Because of the many concomitant health problems, patient are best assessed by a multidisciplinary bariatric surgery team. A coordinator screens BMI and age, and encourages the patient to review covered benefits. The nurse takes a detailed history and collects outside records. The dietician takes a detailed food history and begins the nutrition education process, which include dietary choices and importance of B12, iron, calcium, and folate supplementation. The psychologist screens for underlying disorders that may interfere with understanding expectations and compliance. Exercise or physical therapy can improve physical conditioning. The bariatrician reviews the medical history and coordinates further studies with the primary care physician and specialists, if needed, such as polysomnography (sleep study), echocardiography, or endoscopy. The team meets weekly throughout the evaluative process to monitor the patient’s progress after which a recommendation is made to proceed, or not, with weight loss surgery. The team approach more thoroughly evaluates all aspects of health and together optimizes the patient’s physical and psychological condition for the perioperative period and longer-term weight loss.
Table 1 Conditions Associated with Obesity | |
|---|---|
|
Selective testing should be performed based upon the history and physical examination. The goal of preoperative testing is to identify and modify risk factors that might adversely affect anesthetic care and surgical
outcome. It must be kept in mind that certain testing modalities pose additional risk to the patient, and this risk must be weighed against the potential benefit of improving care based upon the information provided. Testing costs, as well as the potential implications of false-positive or -negative results, must also be considered.
outcome. It must be kept in mind that certain testing modalities pose additional risk to the patient, and this risk must be weighed against the potential benefit of improving care based upon the information provided. Testing costs, as well as the potential implications of false-positive or -negative results, must also be considered.
Laboratory testing is based upon the patient’s medical condition. Hemoglobin is measured when anemia is suspected, which is the case for most female bariatric patients. An electrolyte panel may yield useful information in the presence of diabetes, renal disease, or diuretic use, and coagulation studies are desirable in patients with a history of abnormal bleeding or liver disease. About 20% to 30% of obese patients will have elevated liver function tests (LFT) due to fatty liver, but this has not been shown to alter drug metabolism. Renal clearance may be accelerated due to increased renal blood flow and glomerular filtration rate, a by-product of elevated cardiac output.
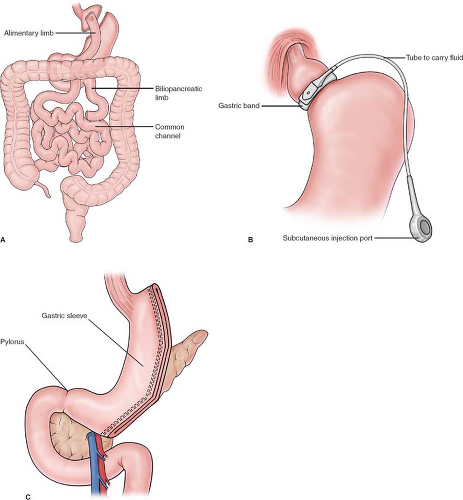 Fig. 1. Most commonly performed weight loss surgery procedures A: Roux-en-Y gastric bypass B: Gastric banding C: Sleeve gastrectomy. |
Traditional markers of cardiac disease, such as exercise tolerance or dyspnea, may be difficult to assess in the severely obese due to concomitant knee and back problems precluding exercise. Signs of cardiac failure such as pulmonary crackles
may be difficult to auscultate with a large body habitus and chronic venous stasis may mask lower extremity edema. Therefore, the threshold to refer for cardiac testing is low. Restrictive effects on lung compliance are common in the morbidly obese. Chest radiographs and pulmonary function tests do not typically yield additional information, unless a disease condition unrelated to obesity is suspected. A baseline arterial blood gas may help detect hypoxic hypoventilation (Pickwickian) syndrome in superobese patients (BMI > 50 kg/m2) with OSA, and may be useful when weaning postoperative ventilatory support.
may be difficult to auscultate with a large body habitus and chronic venous stasis may mask lower extremity edema. Therefore, the threshold to refer for cardiac testing is low. Restrictive effects on lung compliance are common in the morbidly obese. Chest radiographs and pulmonary function tests do not typically yield additional information, unless a disease condition unrelated to obesity is suspected. A baseline arterial blood gas may help detect hypoxic hypoventilation (Pickwickian) syndrome in superobese patients (BMI > 50 kg/m2) with OSA, and may be useful when weaning postoperative ventilatory support.
Fatty liver and nonalcoholic steatohepatitis (NASH) are present in many morbidly obese patients. A large, stiff liver can make access to the stomach more difficult, sometimes requiring additional trocars or conversion to an open procedure. We encourage preoperative weight loss prior to surgery, based on studies showing that a very low energy diet significantly decreases liver volume. Our routine is to ask patients to abstain from soda, desserts, and other sweets, and exercise 30 minutes per day for 1 month. Heavier patients and those with fatty liver noted on preoperative ultrasound are counseled and placed on a 6-week liquid diet prior to surgery.
Airway and Obstructive Sleep Apnea
Perioperative catastrophes of nonsurgical origin in obese patients tend to center around the airway, both in the operating room (OR) and postoperatively. A large percentage of morbidly obese patients have evidence of at least mild OSA, as high as 90% in one study of patients presenting to a bariatric surgery clinic. Various clinical screening tools may be used to detect OSA such as the Berlin questionnaire and the STOP-BANG criteria. The gold standard, however, is polysomnography, the results of which can be used to initiate continuous positive airway pressure (CPAP) therapy. CPAP treatment does not simply allow more restful sleep and relief of daytime somnolence. OSA is also a systemic disease that is an independent risk factor for hypertension, increases the risk of stroke, increases the prevalence of cardiac arrhythmia, and increases the risk of sudden death during sleep. CPAP has been shown to reduce or reverse cardiovascular structural changes induced by severe OSA. In a study of patients with OSA undergoing joint replacement, patients treated preoperatively had fewer perioperative complications. The question remains as to the minimum length of time necessary for preoperative treatment to achieve perioperative risk reduction. Studies measuring the effect of CPAP on endpoints such as correction of abnormal ventilatory drive, improvement in systolic ejection fraction, reduction in hypertension, or reversal of mild pulmonary hypertension have treatment periods of weeks to months. Studies looking specifically at reduction of surgical and anesthetic risk with preoperative CPAP treatment have not been published. The risks of postponing surgery for evaluation and treatment of OSA must be weighed against this imprecise endpoint.
In the American Society of Anesthesiologists (ASA) closed claims study of cases involving the difficult airway, obesity was a factor in 37% of claims related to induction of anesthesia, and in 67% of extubation claims. OSA was present in 28% of extubation claims. Overall, the majority of extubation claims were associated with difficult intubation following induction, obesity, and/or OSA, so this is clearly a risky period for the obese patient. During emergence from anesthesia, it must be ascertained that neuromuscular blockade has been completely reversed. Using respiratory effort alone as evidence of return of full muscle tone can be deceptive, as the diaphragm is one of the most resistant muscles to neuromuscular blockade. In contrast, the pharyngeal muscles recover more slowly. In the obese OSA patient, loss of pharyngeal tone would be expected to exacerbate baseline airway obstruction, particularly in the presence of opiate medications and residual inhalational anesthetic. This combination of factors can lead to loss of the airway following extubation, and should be assiduously avoided. Placing the patient in reverse Trendelenburg (head up) position maximizes lung expansion to assist ventilation during emergence and extubation. In particularly high-risk patients, such as the super morbidly obese (BMI > 50 kg/m2) or severely sleep apneic, we recommend having more than one anesthesia provider immediately available to lend a set of expert hands in the event of unexpected airway difficulty following extubation.
Use of CPAP for early treatment of postoperative hypoxia is well supported in nonbariatric studies. CPAP has been shown to reduce the rate of reintubation, ICU length of stay, and the incidence of pneumonia, infection, and sepsis. In the bariatric anesthesia literature, a recent study by Neligan et al. compared the application of noninvasive positive pressure ventilation (NIPPV), either CPAP or bilevel positive airway pressure (BiPAP), immediately after extubation in the OR versus 30 minutes after extubation in the recovery room, the authors’ standard practice, in a group of morbidly obese patients undergoing either laparoscopic banding or laparoscopic Roux-en-Y gastric bypass. Both groups continued NIPPV overnight. Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were decreased relative to preoperative baseline in both groups, as expected. However, these values were significantly less decreased in the immediate NIPPV group when compared with the standard therapy group at 1 hour and 1 day postoperatively. Unfortunately, the sample size (n = 40) was too small to detect any clinical implications of the intervention, such as a reduced incidence of postoperative respiratory complications. Nonetheless, the study does appear to demonstrate the rapidity with which significant atelectasis develops in the obese population.
Postoperative Monitoring and Pain Management
Another area of uncertainty is which, if any, obese patients require increased postoperative monitoring. At Beth Israel Deaconess Medical Center, patients with severe OSA or BMI > 50 kg/m2 remain in the postanesthesia care unit (PACU) overnight for more intensive nursing care, including monitoring of continuous pulse oximetry. Our criteria are somewhat arbitrary and based upon our clinical experience. Gali et al. have attempted to create more scientifically based criteria for assignment of patients to higher intensity monitored settings. They combined a preoperative OSA screen that stratified patients into two categories, high and low risk, with the presence of recurrent negative events in the PACU. These events included recurrent episodes of apnea, respiratory rate less than 8/minute, oxygen desaturation to less than 90%, and “pain-sedation mismatch,” defined as inadequate pain management in the presence of excess sedation. Patients in the high OSA risk category that also had recurrent PACU events had a 33% chance of respiratory complications in the postoperative period, compared with 2% in the high OSA risk/no recurrent PACU events group and 1% in the low OSA risk/no recurrent PACU events group (Fig. 2). Such predictive criteria may enable more efficient and cost-effective use of limited resources such as PACU and ICU beds by placing only the highest risk patients, the most likely to benefit, in those settings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


