Percutaneous Endoscopic Gastrostomy
Jeffrey L. Ponsky
Benjamin K. Poulose
The endoscopic technique of gastrostomy placement, percutaneous endoscopic gastrostomy (PEG), has permitted long-term gastric access for feeding or decompression without the need for general anesthesia or laparotomy. Reports of comparisons with traditional gastrostomy suggest that the method offers equivalent results with less patient discomfort and at a lower cost. Complications, although consistent with those noted with the open procedure, can be minimized by appropriate patient selection and attention to technical details. Although a number of modifications of the originally described technique have been suggested, the original “pull” method and the closely related “push” approach are the most well-accepted and widely practiced methods.
The most common and appropriate indication for PEG is the need for long-term alimentation in patients unable to eat but with functional gastrointestinal tracts. Such individuals include those with progressive neurologic diseases, stroke victims, individuals with severe psychom otor handicap, patients with head or facial trauma, and patients with oropharyngeal tumors. The method has also been useful in administering unpalatable medications to children, in the administration of nighttime supplemental feedings to patients with inflammatory bowel disease, as a route for returning external biliary drainage to the gastrointestinal tract, and in providing gastric decompression in those patients with carcinomatosis or radiation enteritis.
Contraindications to PEG are few, but important to consider in minimizing morbidity and mortality of this procedure. The procedure should be avoided in patients with massive ascites, bleeding diatheses, or severe malnutrition as tract formation is impaired and leakage may result. Multiple system organ failure or systemic sepsis should be corrected prior to placement of a PEG, as infectious complications are more likely to occur. Feedings may be provided by the nasoenteric route until these problems are resolved. Also, the performance of PEG in patients with very limited life expectancy (i.e., <30 days) is meddlesome and costly, contributing to the high in-hospital mortality reported following the procedure. PEG has not proven particularly useful in patients with aspiration secondary to severe gastroesophageal reflux, and in some cases it may exacerbate the problem.
Technique
The patient is prepared by fasting overnight or by withholding enteral feedings for 6 to 8 hours prior to the procedure. Preprocedural imaging, if available, is reviewed for anatomic alterations such as a large hiatal hernia. Intravenous access is established for the administration of sedation and wound infection prophylaxis. A preoperative intravenous antibiotic, usually a cephalosporin, is administered to reduce the incidence of wound infection in the abdominal wall surrounding the tube. Swabbing of the oropharynx with an antiseptic solution can help reduce bacterial counts in the mouth and thus may help protect against wound infection. Moderate sedation using an intravenous opioid analgesic and benzodiazepine is usually sufficient for PEG insertion. Alternatively, deep sedation with intravenous propofol can be used in concert with the anesthesia team. Respiration, blood pressure, pulse rate, and oxygen saturation are monitored.
The patient is placed on the endoscopy table in the supine position. Although this position is most appropriate for the performance of PEG, it predisposes to the accumulation of secretions in the patient’s oropharynx and may lead to aspiration. Therefore, frequent evacuation of this material by suctioning is imperative. Abdominal hair in the upper abdomen is clipped and the skin cleaned with a preparatory solution, and draped in a sterile manner. The procedure is commenced with the introduction of the gastroscope into the patient’s esophagus. This can be somewhat difficult with the
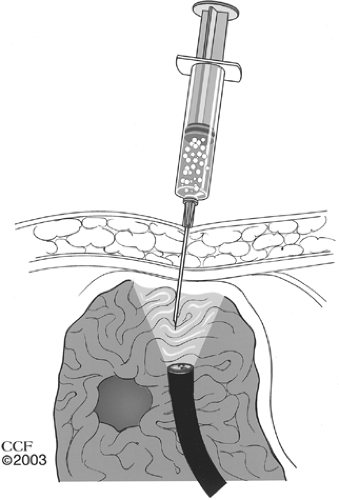
patient in the supine position and may be facilitated by slightly turning the patient to the left for passage of the instrument. The scope is then advanced into the stomach and the room lights are dimmed. A rapid but thorough inspection of the stomach and duodenum should be performed to rule out pathology or pyloric obstruction. The instrument is then pulled back into the gastric body and turned anteriorly as the assistant inspects the external aspect of the abdominal wall, looking for transillumination. Transillumination of the abdominal wall by the light from the endoscope can help localize the optimal area for gastric lumenal access. Finger palpation of the abdominal wall may reveal a point of light when gentle pressure is applied. Once the point of brightest transillumination is identified, continued finger pressure is applied to this site by the assistant, and this should create a clear and prominent indentation of the gastric wall that is visible to the endoscopist. Multiple sites may be palpated until the best transillumination and gastric indentation are achieved. Alternate palpation of two separate fingers adjacent to each other with visible confirmation of two separate indentations on endoscopic examination can also help identify an area for safe gastric access. Extra time spent at this stage of the procedure is well worth the effort and will help reduce inadvertent puncture of adjacent organs (Fig. 1).
patient in the supine position and may be facilitated by slightly turning the patient to the left for passage of the instrument. The scope is then advanced into the stomach and the room lights are dimmed. A rapid but thorough inspection of the stomach and duodenum should be performed to rule out pathology or pyloric obstruction. The instrument is then pulled back into the gastric body and turned anteriorly as the assistant inspects the external aspect of the abdominal wall, looking for transillumination. Transillumination of the abdominal wall by the light from the endoscope can help localize the optimal area for gastric lumenal access. Finger palpation of the abdominal wall may reveal a point of light when gentle pressure is applied. Once the point of brightest transillumination is identified, continued finger pressure is applied to this site by the assistant, and this should create a clear and prominent indentation of the gastric wall that is visible to the endoscopist. Multiple sites may be palpated until the best transillumination and gastric indentation are achieved. Alternate palpation of two separate fingers adjacent to each other with visible confirmation of two separate indentations on endoscopic examination can also help identify an area for safe gastric access. Extra time spent at this stage of the procedure is well worth the effort and will help reduce inadvertent puncture of adjacent organs (Fig. 1).
The “safe tract” technique, described by Foutch et al., has been developed to help minimize the risk of visceral injury during PEG placement. This approach is especially useful in obese patients where other methods of gastric lumenal access localization are inadequate. In this method, the best site for puncture is initially localized by transillumination and finger pressure, as previously described. Then, using a syringe half-filled with local anesthetic in its barrel, a small-caliber needle is inserted into the skin at the proposed spot and suction is applied to the plunger. The syringe is slowly advanced into the stomach until the needle is seen to enter the gastric lumen endoscopically and air is seen to bubble into the syringe barrel externally. Should air appear in the barrel prior to the appearance of the needle in the gastric lumen, one may assume that there is a loop of bowel interposed between the stomach and the abdominal wall. The needle is removed and an alternative site is selected and tested in the same manner. In this way, the possibility of a fistulous communication is minimized (Fig. 2). A few milliliters of local anesthetic are infiltrated into the skin at the selected site, and a 5-mm incision is made through the skin and into the subcutaneous tissue.
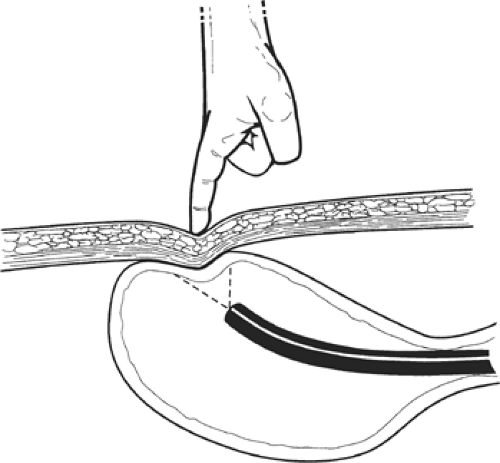 Fig. 1. The site for gastric lumenal access should be carefully selected, using transillumination of the abdominal wall and gastric indentation with finger pressure as a guide. |
While the assistant anesthetizes the abdominal wall skin, the endoscopist uses a polypectomy snare, passed through the biopsy channel of the endoscope, to surround the area of gastric indentation. When the endoscopist is ready, the assistant passes a needle cannula with an outer plastic sheath through the skin incision and into the stomach. This maneuver is done swiftly to minimize displacement of the gastric wall away
from the abdominal wall. The open polypectomy snare in the gastric lumen will surround the cannula and is tightened around it. The inner needle is then removed and a long suture or wire is threaded through the cannula into the stomach. After several centimeters of the suture have entered the stomach, the snare is slightly loosened, the cannula pulled back a bit, and the snare retightened around the suture itself (Fig. 3). The gastroscope is then withdrawn from the patient’s mouth, pulling the suture along with it.
from the abdominal wall. The open polypectomy snare in the gastric lumen will surround the cannula and is tightened around it. The inner needle is then removed and a long suture or wire is threaded through the cannula into the stomach. After several centimeters of the suture have entered the stomach, the snare is slightly loosened, the cannula pulled back a bit, and the snare retightened around the suture itself (Fig. 3). The gastroscope is then withdrawn from the patient’s mouth, pulling the suture along with it.
The two most frequently used techniques for PEG are the pull and the push methods. Typically, 20 French PEG tubes are inserted. In the pull method, the gastrostomy tube is affixed to the suture exiting the patient’s mouth and pulled down through the esophagus and into the stomach, its end emerging from the abdominal wall (Fig. 4). In the push method, a guidewire instead of a suture is used and the tube is threaded over the wire and pushed down the esophagus and into the stomach as tension is applied to both ends of the guidewire (Fig. 5). In each method, when the tube emerges from the abdominal wall it is grasped and pulled upward. The gastroscope is reintroduced to monitor the position of the head of the catheter (i.e., the intralumenal bumper) and to ensure that it comes to lie in contact with the gastric mucosa.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree