39
Penicillins, Cephalosporins, and Other β-Lactam Antibiotics
This chapter will be most useful after having a basic understanding of the material in Chapter 53, Penicillins, Cephalosporins, and Other β-Lactam Antibiotics in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition contains:
• A detailed discussion of the mechanisms of action and resistance of the penicillins and cephalosporins
• A detailed discussion of each of the penicillins and cephalosporins, including their pharmacokinetics and therapeutic uses in specific infections
• Figure 53-3 which shows a comparison of the structure and composition of gram-positive and gram-negative bacterial cell walls
• Table 53-1 which shows the chemical structures and major properties of the various penicillins
• Table 53-2 which shows the chemical structures and dosage forms of selected cephalosporins and related compounds
• Table 53-3 which shows the cephalosporin generations and the useful spectrum of each generation
LEARNING OBJECTIVES
 Understand the mechanisms of action of the penicillins, cephalosporins, and other β-lactam antibiotics.
Understand the mechanisms of action of the penicillins, cephalosporins, and other β-lactam antibiotics.
 Understand the mechanisms of resistance of the penicillins, cephalosporins, and other β-lactam antibiotics.
Understand the mechanisms of resistance of the penicillins, cephalosporins, and other β-lactam antibiotics.
 Describe the therapeutic effects of the penicillins, cephalosporins, and other β-lactam antibiotics.
Describe the therapeutic effects of the penicillins, cephalosporins, and other β-lactam antibiotics.
 Describe the untoward effects and contraindications of the penicillins, cephalosporins, and other β-lactam antibiotics.
Describe the untoward effects and contraindications of the penicillins, cephalosporins, and other β-lactam antibiotics.
MECHANISMS OF ACTION AND RESISTANCE OF PENICILLINS, CEPHALOSPORINS, AND β-LACTAMASE INHIBITORS
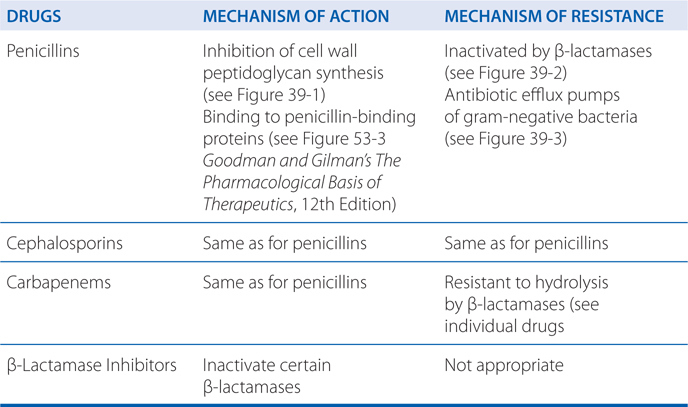
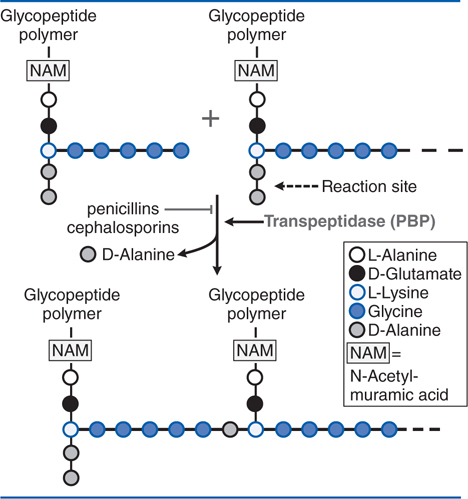
FIGURE 39-1 Action of β-lactam antibiotics in Staphylococcus aureus. The bacterial cell wall consists of glycopeptide polymers (a NAM-NAG amino-hexose backbone) linked via bridges between amino acid side chains. In S. aureus, the bridge is (Gly)5–D-Ala between lysines. The cross-linking is catalyzed by a transpeptidase, the enzyme that penicillins and cephalosporins inhibit. NAM, N-acetyl-muramic acid; NAG, N-acetyl-glucosamine.
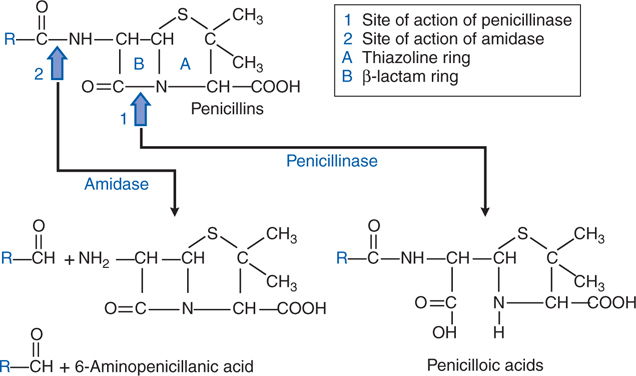
FIGURE 39-2 Structure of penicillins and products of their enzymatic hydrolysis.
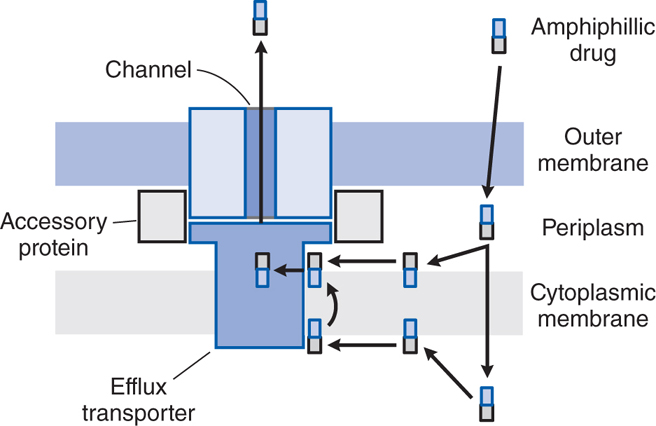
FIGURE 39-3 Antibiotic efflux pumps of gram-negative bacteria. Multidrug efflux pumps traverse both the inner and outer membranes of gram-negative bacteria. The pumps are composed of a minimum of 3 proteins and are energized by the proton motive force. Increased expression of these pumps is an important cause of antibiotic resistance. (Reprinted with permission from Oxford University Press. Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin Infect Dis, 1998, 27[suppl I]:S32-S41. © 1998 by the Infectious Diseases Society of America. All rights reserved.)
DRUGS INCLUDED IN THIS CHAPTER
Penicillins
Penicillin G
Penicillin V
Methicillin
Oxacillin (BACTOCILL)
Nafcillin
Ampicillin
Amoxicillin
Carbenicillin
Ticarcillin
Mezlocillin
Piperacillin
Cephalosporins
Cephazolin (ANCEF, KEFZOL, others)
Cephalexin (KEFLEX, others)
Cefadroxil (DURACEF)
Cephradine (VELOCEF)
Cefuroxime (ZANACEF)
Cefuroxime axetil (CEFTIN)
Cefprozil (CEFZIL)
Cefmetazole (ZEFAZONE)
Loracarbef (LORABID)
Cefotaxime (CLAFORAN)
Ceftriaxone (ROCEPHIN)
Cefdinir (OMNICEF)
Cefditoren pivoxil (SPECTRACEF)
Ceftibuten (CEDAX)
Cefpodoxime proxetil (VANTIN)
Ceftizoxime (CEFIZOX)
Cefoperazone (CEFOBID)
Ceftazidime (FORTAZ, others)
Cefepime (MAXIPINE)
Other β-Lactam Antibiotics
Imipenem (PRIMAXIN)
Meropenem (MERREM)
Doripenem (DORIBAX)
Ertapenem (INVANZ)
Aztreonam (AZACTAM)
β-Lactamase Inhibitors
Clavulanic acid (AUGMENTUM, combination with amoxicillin; TIMENTIN, combination with ticarcillin)
Sulbactam (UNASYN, combination with ampicillin)
Tazobactam (ZOSYN, combination with piperacillin)
• Structural differences in penicillin-binding proteins (PBPs)
• Decreased affinity for PBPs
• Inability of antibiotic to penetrate to site of action
• Efflux pumps that extrude antibiotic (see Figure 39-3)
• Enzymatic (β-lactamase) destruction of antibiotic (see Figure 39-2)
A 56-year-old man is brought to hospital with a productive cough and temperature of 103°F. A chest X-ray reveals pneumonia in the left lower lobe. After obtaining sputum and blood cultures he is started on an intramuscular injection of penicillin G. His symptoms do not improve over the next 24 hours and the cultures return showing S. pneumoniae not sensitive to penicillin.
a. What is the mechanism of action of penicillin to sensitive bacteria?
The cell walls of bacteria are essential for their normal growth and development. Peptidoglycan is a heteropolymeric compound of the cell wall that provides rigid mechanical stability by virtue of its highly cross-linked latticework structure (see Figure 39-1). Figure 53-3 in Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition shows a comparison of the structure and composition of gram-positive and gram-negative cells. In the synthesis of the bacterial cell wall, the terminal glycine residue of the pentaglycine bridge is linked to the fourth residue of the pentapeptide (D-alanine), releasing the fifth residue (also D-alanine). It is this step in peptidoglycan synthesis that is inhibited by the β-lactam antibiotics and glycopeptide antibiotics such as vancomycin (see Chapter 41). There are additional targets for the actions of penicillins and cephalosporins that are collectively termed penicillin-binding proteins (PBP). The PBPs vary in their affinities for different β-lactam antibiotics, although the interactions eventually become covalent.
CLASSES OF β-LACTAMASE ENZYMES
• Class A—includes the extended-spectrum β-lactamases (ESBLs) that degrade penicillins, some cephalosporins, and, in some instances, carbapenems.
• Class B—are Zn2+-dependent enzymes that destroy all β-lactams except aztreonam.
• Class C—active against cephalosporins.
• Class D—includes cloxacillin-degrading enzymes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree