Pediatric Frozen Section
Ajit S. Paintal
Aliya N. Husain
At University of Chicago Medical Center, the most frequent indications for intraoperative consultation in children are bone tumors and brain tumors which are discussed in Chapters 4 and 15, respectively. Approximately 9% of our pediatric intraoperative consultations are performed for the evaluation of Hirschsprung disease; the remaining consultations are done for tumors. Evaluation of an immunocompromised child is a rare indication for frozen section and is discussed elsewhere in this volume. Our overall frozen section concordance rate (95%) and deferral rate (11%) are roughly comparable to what has been reported in the literature at other institutions (1,2).
Introduction: Pediatric Solid Tumors
Relative to adults, malignancies in children are uncommon, accounting for approximately 7,000 cases per year in the United States. Hematopoietic neoplasms are the largest single group of cancers leaving a mixture of solid tumors to account for approximately 60% of all cases. The spectrum of malignancy in infants and children differs markedly from that seen in the adult population and most of these patients receive treatment at specialized tertiary centers. Because of these factors, most nonpediatric pathologists do not accrue substantial amounts of experience with these diseases.
Over the past several decades, outcomes in pediatric cancer have improved dramatically. Improved accuracy in diagnosis, recognition of biologically significant prognostic features, and tailored therapy using multiple modalities have all contributed to the improved outlook. Successful treatment of these diseases hinges on the correct initial classification of the malignancy and recognition of significant morphologic (e.g., anaplasia in Wilms tumors) and genetic (e.g., N-myc amplification in neuroblastoma) prognostic factors.
Pediatric Solid Tumors: Major Intraoperative Questions
Because of the important role that molecular and genetic testing play in the diagnosis of pediatric tumors, a precise diagnosis is often not possible intraoperatively. Definitive surgery and therapy are properly based only
upon thorough histologic examination of fixed tissues and interpretation of ancillary data. The frozen section in this setting may address the basic question of the benign versus malignant nature of a tumor so that a catheter may be placed for future chemotherapy. A malignant diagnosis may also initiate staging bone marrow biopsies which can be performed while the patient is under anesthesia.
upon thorough histologic examination of fixed tissues and interpretation of ancillary data. The frozen section in this setting may address the basic question of the benign versus malignant nature of a tumor so that a catheter may be placed for future chemotherapy. A malignant diagnosis may also initiate staging bone marrow biopsies which can be performed while the patient is under anesthesia.
A second use for frozen section in pediatric tumors is in the assessment of margin status and extent of disease. Margins are critically important in most pediatric tumors, and in the case of hepatoblastoma, complete resection is essential for disease cure (3). In most cases, 1- to 2-mm margins are sufficient, particularly when operating in the head and neck regions (4).
Last, frozen section may facilitate triage and assessment of adequacy of a biopsy. In this scenario, requesting an intraoperative consultation serves to bring the specimen to the pathologist’s attention allowing the viability of the tissue to be assessed and the tissue to be apportioned for cytogenetics, electron microscopy, and molecular studies (5). In addition, cytologic preparations (imprints) can be made at this time which are often a valuable adjunct in arriving at the final diagnosis (6).
Pediatric Solid Tumors and Frozen Section Interpretation
As in other settings, the nature of the question being posed usually determines how the intraoperative consultation is performed. In the case of determining adequacy or whether a lesion is benign or malignant, intraoperative cytology is invaluable, especially if material is limited. In a single series, intraoperative cytology to evaluate masses in pediatric patients with knowledge of the clinical and radiographic data had an accuracy rate ranging from 93% to 98% among three observers and was particularly effective in the case of small round blue cell tumors allowing general classification as such in more than 95% of cases. Performing a concurrent frozen section and intraoperative evaluation of histology only increased the accuracy rates by 0% to 3%, depending on the observer (7). If material is limited, intraoperative cytology alone is almost always sufficient to determine if diagnostic tissue is present and to classify a lesion as benign or malignant.
Particularly in the case of small biopsies which are frequently crushed and subject to fixation artifact, cytologic touch preparations are useful in that the cellular morphology is often extremely well preserved with minimal artifactual distortion. Employing cytology in small biopsies is also useful in that it allows evaluation of a complementary set of morphologic data when combined with histology (both permanent sections and frozen sections).
Lymphoma frequently enters the clinical differential diagnosis for masses in the pediatric population and overlap morphologically with other small round blue cell tumors. In contrast to adults where neoplasms composed of small lymphocytes are common, pediatric lymphomas are typically
high grade. The most common pediatric non-Hodgkin lymphomas are lymphoblastic lymphoma, Burkitt lymphoma, and large cell lymphoma (8).
high grade. The most common pediatric non-Hodgkin lymphomas are lymphoblastic lymphoma, Burkitt lymphoma, and large cell lymphoma (8).
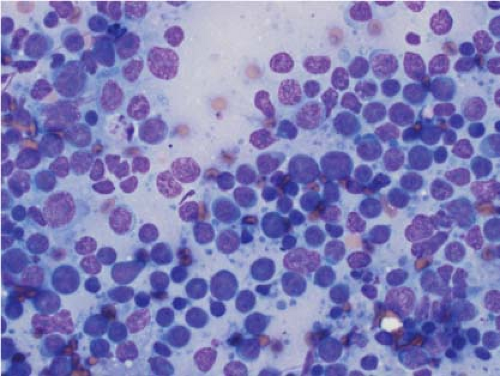
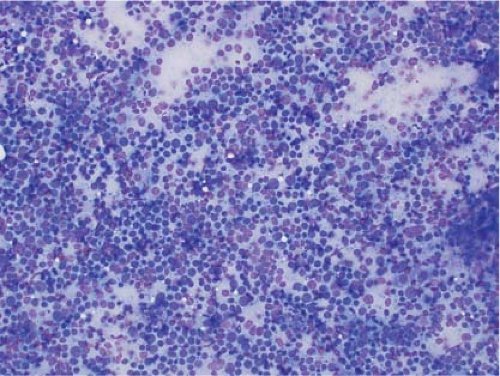
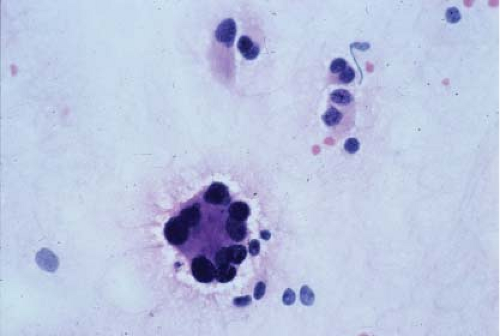
In the context of intraoperative touch preparations, identifying a lesion as lymphoma is useful in that it may prompt triage of material for flow cytometry. Two of the most useful features that identify a lesion as lymphoid in nature are lymphoglandular bodies and the quality of the cytoplasm. Lymphoglandular bodies are small fragments of lightly azurophilic cytoplasm measuring 2 to 7 μm. While occasional lymphoglandular bodies may be seen in many tumor types, the presence of numerous lymphoglandular bodies is relatively specific for a lymphoid tumor and seen in greater than 90% of cases. Of note, lymphoglandular bodies may be seen in any lymphoid proliferation and cannot be used to distinguish a reactive lymphoid population from lymphoma. Less specific features associated with lymphoma in cytologic preparations are the degree of cohesion and quality of the cytoplasm. Lymphoid cells are almost always dyscohesive and generally have a peripheral rim of dark blue cytoplasm with relative pallor adjacent to the nucleus (9,10) (Figs. 7.1 and 7.2).
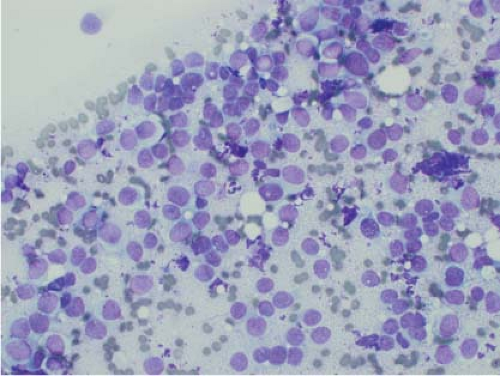
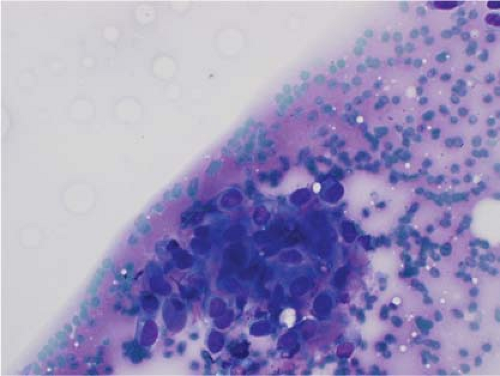
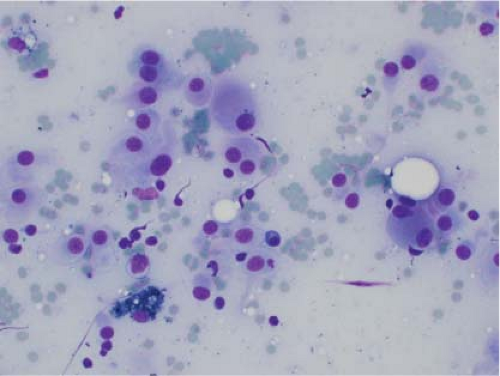
Ewing sarcoma is another relatively common small round blue cell tumor that may present at both skeletal and extraskeletal sites. While the cytologic findings in touch preps of this tumor are classic and highly suggestive of the diagnosis in many cases, Ewing sarcoma is characterized by a wide morphologic spectrum and, as such, the diagnosis rests heavily upon immunohistochemical findings and the demonstration of the t(11;22) translocation. In contrast to lymphoma, a greater degree of cohesion is
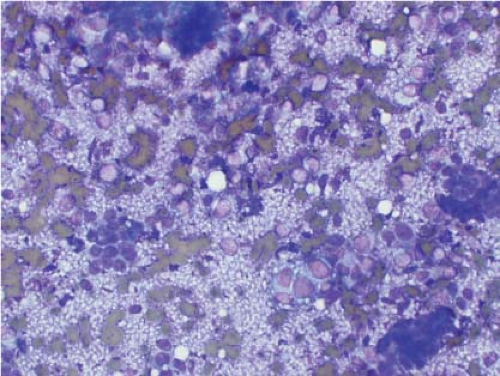
seen in Ewing sarcoma with tight clusters of cells admixed with a dispersed single cell population. The cells in typical cases usually range from intermediate to large in size and frequently contain cytoplasmic glycogen. Glycogen may be visualized either in the form of cytoplasmic vacuoles or as reticulated, web-like background material (“tigroid” background). The nuclei, while typically round with even chromatin, may have prominent nucleoli and a coarse texture in more poorly differentiated forms. A degree of nuclear molding may be seen but is generally not as pronounced as that seen in neuroendocrine carcinomas. A biphasic cell population, consisting of viable cells and smaller, degenerated cells with pyknotic nuclei, is often noted (11,12,13) (Figs. 7.3 and 7.4).
seen in Ewing sarcoma with tight clusters of cells admixed with a dispersed single cell population. The cells in typical cases usually range from intermediate to large in size and frequently contain cytoplasmic glycogen. Glycogen may be visualized either in the form of cytoplasmic vacuoles or as reticulated, web-like background material (“tigroid” background). The nuclei, while typically round with even chromatin, may have prominent nucleoli and a coarse texture in more poorly differentiated forms. A degree of nuclear molding may be seen but is generally not as pronounced as that seen in neuroendocrine carcinomas. A biphasic cell population, consisting of viable cells and smaller, degenerated cells with pyknotic nuclei, is often noted (11,12,13) (Figs. 7.3 and 7.4).
Rhabdomyosarcoma may be seen in the pediatric population and predominantly affects the head and neck or extremities. While embryonal rhabdomyosarcoma is composed of spindle cells, alveolar rhabdomyosarcoma is a small round blue cell tumor and may overlap considerably with other entities in this category. The typical smear demonstrates loosely cohesive groups as well as individual tumor cells. Large rhabdoid forms with eccentric nuclei and multiple nuclei, classically peripherally placed and forming a ring, are relatively specific for this diagnosis. While cytoplasmic vacuolization, similar to that seen in Ewing sarcoma, is common in alveolar rhabdomyosarcoma, the cytoplasm often has a denser quality and the cell borders are well defined. The nuclei are typically irregular and hyperchromatic. Cross-striations, frequently cited as a useful diagnostic feature in rhabdomyosarcoma, are almost never seen (14,15,16).
Neuroblastomas most frequently present as an abdominal mass in younger children with peak incidence in the first 3 years of life. The most characteristic feature of this tumor is the presence of a fibrillary background containing neuropil. Homer-Wright pseudorosettes are seen in classic cases. The cells are round with scant cytoplasm and the nuclei in most cases are round with a granular chromatin pattern. The degree to which the diagnostic morphologic features of neuroblastoma are developed rests heavily on the level of maturity. Undifferentiated and poorly differentiated neuroblastomas generally display more primitive nuclear features and lack neuropil in cytologic preparations. In contrast, neuroblastic differentiation is more apparent in cases of differentiating neuroblastoma. More mature forms may also contain occasional larger eosinophilic cells with large nuclei and prominent nucleoli (ganglion cell differentiation) (17,18,19) (Fig. 7.5).
Desmoplastic small round cell tumor typically involves the abdominal cavity in young adult males but may occur in females and at other sites as well. A useful feature in distinguishing this entity from other small round blue cell tumors in cytologic preparations is the presence of fragments of metachromatic (magenta in the Diff-Quick stain) stroma. Cell clusters frequently show prominent nuclear molding and individual cells are notable for delicate vacuolated cytoplasm and finely granular chromatin. Given the substantial overlap with Ewing sarcoma, morphologic distinction of these entities is not reliable (20,21).
Osteosarcoma, particularly the small cell variant of osteosarcoma, may present as a small round blue cell tumor at both skeletal and extraskeletal sites. In touch preparations, osteosarcoma generally exhibits brisk mitotic
activity and karyorrhexis. Rosette formation is not seen. The tumor cells are cohesive and contain varying amounts of blue cytoplasm. In contrast to Ewing sarcoma and most other small round blue cell tumors, spindling may be seen. The diagnostic hallmark of this tumor in cytologic preparations is the identification of osteoid which appears as delicate, lacy metachromatic material, oftentimes surrounding individual tumor cells (22,23) (Fig. 7.6).
activity and karyorrhexis. Rosette formation is not seen. The tumor cells are cohesive and contain varying amounts of blue cytoplasm. In contrast to Ewing sarcoma and most other small round blue cell tumors, spindling may be seen. The diagnostic hallmark of this tumor in cytologic preparations is the identification of osteoid which appears as delicate, lacy metachromatic material, oftentimes surrounding individual tumor cells (22,23) (Fig. 7.6).
Melanoma is protean in its morphologic appearance and may enter into the differential diagnosis of a small round blue cell tumor. Cytologic preparations are characterized by marked cellularity and dyscohesion. Individual cells may have scant to abundant cytoplasm with both spindled and epithelioid forms. Plasmacytoid morphology is frequently well developed to the extent that nuclei appear to be in contact with the cell membrane. The cytoplasm may contain melanin pigment which is diagnostic when present. Cytoplasmic vacuolization is also common. The nuclei may be bland or exhibit wild pleomorphism. A morphologic finding that is highly suggestive of melanoma when present is binucleated cells with widely spaced nuclei at opposite ends of the cell. Clear cell sarcoma (formerly designated malignant melanoma of soft parts) cannot be reliably distinguished from malignant melanoma based on morphologic and immunohistochemical findings alone and requires cytogenetic demonstration of the t(12;22) translocation or its variants to confirm the diagnosis. Helpful features which favor clear cell sarcoma over melanoma include nuclear monotony (characteristic of many translocation-associated tumors) and involvement of the deep soft tissue of the extremities (24) (Figs. 7.7 and 7.8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree