Bone and Soft Tissue
Michelle O’leary
Anthony G. Montag
Introduction
The relative rarity of bone lesions, especially tumors, makes requests for frozen section uncommon. Surgical pathologists may feel uncomfortable in assessing these lesions intraoperatively if major treatment decisions are to be made. It may even occur to pathologists that such requests are unreasonable. However, the recent clinical advances in limb-sparing sarcoma management have created a rationale for intraoperative assessment. The differential diagnosis of bone lesions is considered from clinical, radiologic, and pathologic perspectives, and a number of cases are provided to illustrate common diagnostic problems and pitfalls.
Most bone lesions have typical clinical presentations and classical radiologic features. The pathologist who is able to correlate this information with the morphologic features will avoid making errors in pathologic diagnosis by frozen section. If the clinical, radiologic, and pathologic impressions are concordant, then the pathologist can be reasonably reassured that the diagnosis is correct. However, if any of the three factors is discordant, the diagnosis should be deferred until a permanent section is obtained. It is important to emphasize that the treatment algorithm in the limb salvage era is somewhat backward to what would be ordinarily anticipated.
A diagnosis of malignancy made from a frozen section will result in closing of the wound to await permanent sections and confirmation of the diagnosis. After appropriate staging, the definitive limb salvage resection will be preceded by neoadjuvant therapy. If the permanent section and evaluation of nonfrozen tissue reveals a benign diagnosis, the lesion will be re-explored, curetted, and packed with cement or bone chips. The error and possible embarrassment to the pathologist aside, the patient will require a second procedure and the final clinical outcome will be a good one for the patient (1).
A benign diagnosis allows the surgeon to curette and pack the lesion directly. If the lesion is in fact benign, then the therapy is complete. If, however, the final interpretation of the frozen and nonfrozen tissues is that the lesion is malignant, then the surgical site has been contaminated and is unsuitable for limb salvage operation. A local
recurrence rate of 83% in erroneously diagnosed osteosarcoma treated by limb salvage operation suggests that the patient will require an amputation (2).
Therefore, in conducting intraoperative consultations and analyzing frozen sections for bone lesions, especially tumors, the message for the pathologist is a conservative one. With experience, definitive diagnoses and appropriate treatment can be instituted. However, under any circumstances, if there is a diagnostic question, the interpretation should be deferred for definitive evaluation.
Clinical Information
Most patients with bone lesions present in a characteristic age range of two to three decades, which can quickly narrow down the clinical differential diagnosis (3) (e-Fig. 4.1). For example, both chondroblastoma and giant cell tumor occur in younger individuals; but chondroblastoma typically occurs prior to or near the time of epiphyseal closure in the teenage years, while giant cell tumor tends to occur later. Osteosarcoma is the most common malignant bone tumor that occurs in childhood, but it also occurs in older patients.
A history of sickle cell anemia or other potential cause of bone infarct raises the possibility of malignant fibrous histiocytoma of bone. A history of Paget disease of bone or prior radiation therapy to the region also raises the possibility of secondary sarcoma.
A previous history of nonosseous malignancy is particularly helpful. Metastatic lesions to bone are far more common than primary bone lesions: 10% to 15% of patients with metastases of unknown primary tumors present with bone lesions (4) and up to 30% of skeletal metastases constitute the first clinical evidence of a malignancy. As a general rule, any poorly marginated lytic bone lesion in a patient older than 40 years should be suspected to be a metastasis until proven otherwise.
Bone ranks number three (behind lung and liver) as one of the most common sites of clinical metastasis. In autopsy studies, it is the most frequent site of metastasis; up to 60% of patients who die of carcinoma are found to have bone metastases (5). The most common malignancies that metastasize to bone are lung, breast, prostate, kidney, and thyroid malignancies. Although metastases are very uncommon in children, lesions that do metastasize to bone in this population include neuroblastoma, rhabdomyosarcoma, and clear cell sarcoma of the kidney. Metastatic lesions frequently undergo internal fixation, and frozen section confirmation is recommended to avoid the placement of hardware in a primary bone tumor, contaminating the area and obviating the option for limb salvage surgery on an early stage primary.
Fractures may complicate benign or malignant bone tumors and metastases, or may be entirely traumatic in origin but mimic a tumor radiographically. Benign tumors in the small bones of the hand are particularly prone to pathologic fractures (6). Pathologic fractures are uncommon in children, but
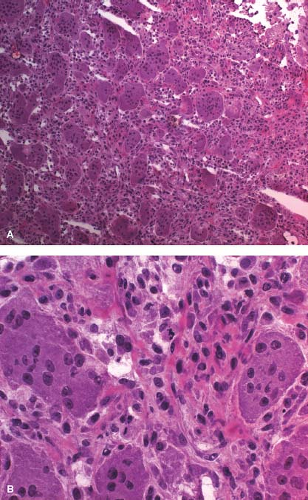
most frequently occur in association with unicameral bone cyst, nonossifying fibroma, fibrous dysplasia, aneurysmal bone cyst (ABC), osteosarcoma, and Ewing sarcoma (7,8,9). In an adult older than 40 years, pathologic fracture should always raise the suspicion of metastasis. Care must be taken not to interpret fracture callus as a malignant neoplasm (10). Fracture callus can present with many of the elements of osteosarcoma including sheets of large osteoblasts, mitotic activity, woven bone, immature cartilage matrix, and cellular fibroblastic proliferation (e-Fig. 4.2A–E). Table 4.1 indicates the morphologic stages of a healing fracture. A comparison of histologic fracture with osteosarcoma is given in e-Figure 4.3. The overall zonal architecture, progressive maturation, broad woven bone trabeculae, and osteoblast rimming are important histologic clues that the lesion is a fracture.
most frequently occur in association with unicameral bone cyst, nonossifying fibroma, fibrous dysplasia, aneurysmal bone cyst (ABC), osteosarcoma, and Ewing sarcoma (7,8,9). In an adult older than 40 years, pathologic fracture should always raise the suspicion of metastasis. Care must be taken not to interpret fracture callus as a malignant neoplasm (10). Fracture callus can present with many of the elements of osteosarcoma including sheets of large osteoblasts, mitotic activity, woven bone, immature cartilage matrix, and cellular fibroblastic proliferation (e-Fig. 4.2A–E). Table 4.1 indicates the morphologic stages of a healing fracture. A comparison of histologic fracture with osteosarcoma is given in e-Figure 4.3. The overall zonal architecture, progressive maturation, broad woven bone trabeculae, and osteoblast rimming are important histologic clues that the lesion is a fracture.
Table 4.1 Stages of Fracture Maturation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In general, most symptomatic bone lesions present with pain. Cartilaginous tumors of the long bones which present with pain are more likely to be chondrosarcoma, whereas enchondromas are typically asymptomatic. A history of penetrating wound, immunosuppression, sickle cell disease, or previous sepsis raises the possibility of osteomyelitis, which nearly always presents with an elevated sedimentation rate and usually with an elevated C-reactive protein (11). While primary bone tumors are most often solitary, multifocality indicates either fibrous dysplasia, vascular tumor, a congenital or syndromic condition or metastatic disease (Table 4.2).
Table 4.2 Differential Diagnosis of Multifocal Bone Lesions | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Radiographic Impression
The radiograph is the gross impression for a bone biopsy, and at the time of frozen section analysis, it is advisable to review the radiograph along with the clinician or at least have the interpretation of a musculoskeletal radiologist available. The radiographic features allow formulation of a differential diagnosis based on the area of the skeleton (extremities, axial, craniofacial), the anatomic location within the bone (epiphyseal, metaphyseal, diaphyseal), and the position within the bone (central, eccentric, cortical, juxtacortical) (12). Many of the bone lesions that occur in the extremities have a predilection for characteristic sites (Table 4.3); for example, giant cell tumors occur mostly around the knee, enchondromas are common in the bones of the hands and feet, and adamantinoma nearly always occurs in the tibia.
In general, a slow-growing bone lesion will display an abrupt margin of transition with the surrounding bone, usually with a rim of sclerotic
host bone. Malignant lesions are more likely to have an indistinct transition and a “moth-eaten” appearance, while lacking a rim of sclerosis.
host bone. Malignant lesions are more likely to have an indistinct transition and a “moth-eaten” appearance, while lacking a rim of sclerosis.
Table 4.3 Extremity Bone Lesions by Location | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cortical destruction and periosteal extension are characteristic of malignant tumors. The infiltration of the periosteal tissues is associated with thickening and elevation of the periosteum and, when associated with mineralization, appears as Codman triangle. Expansile tumors such as giant cell tumor and ABC may markedly distort the cortex, but usually retain a thin shell of reactive bone. Soft-tissue extension is frequently difficult to see on a standard radiograph, but is more easily seen on CT or MRI scans.
The pattern of mineralization also gives clues for tumor diagnosis. Benign bone-forming tumors tend to have a uniform distribution of osteoid matrix, while osteosarcoma tends to be less homogenous. Fibrous dysplasia has a “ground-glass” appearance on radiographs because of the numerous and fairly evenly spaced bone spicules. Enchondromas tend to have ossification and calcification at the edge of lobules, and they usually present a pattern of interlacing arcs of matrix on radiographs.
During definitive excision, the marrow at the bony margin is frozen or subjected to imprint preparations so as to assess the presence of tumor. The marrow in long bones is usually fatty; however, use of colony-stimulating factors during chemotherapy may result in a disconcertingly hypercellular marrow. The MRI is extremely accurate and helpful in evaluating the extent of marrow involvement (13), and in practice, at our institution we have not seen a positive marrow margin on frozen section for more than a decade.
Important Differentials Through Case Studies
Epiphyseal Lesions
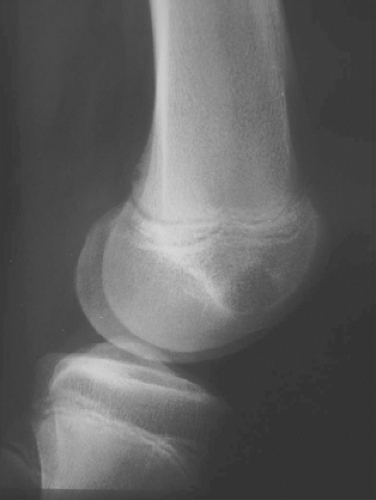
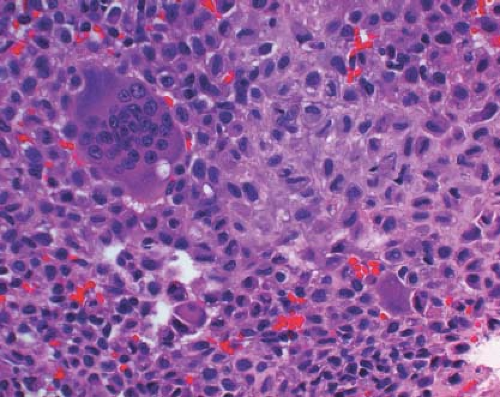
A 16-year-old adolescent boy presented with knee pain 6 weeks after an athletic injury. Imaging showed a well-delineated, lytic, and partially cystic lesion with variable calcification involving the epiphysis of the distal femur (Fig. 4.1). An imprint of a curetted specimen demonstrated polygonal, mononuclear cells, mildly atypical in appearance with nuclear folds (Fig. 4.2). The frozen section showed a “chicken-wire” pattern of calcification of the matrix (Fig. 4.3) and mononuclear stromal cells with scattered osteoclast-like giant cells (Fig. 4.4). These features are diagnostic of chondroblastoma and are consistent with the clinical and radiographic features. The differential diagnosis of an epiphyseal lesion includes chondroblastoma, giant cell tumor, and clear cell chondrosarcoma. Chondroblastoma occurs in a younger age group, typically before the closure of the epiphysis, and has a characteristic matrix. The giant cell tumor typically has a diffuse distribution of giant cells (Fig. 4.5), but this feature can also be seen in areas of chondroblastoma. The nucleus of the mononuclear stromal cell of chondroblastoma is folded, hyperchromatic, smudgy, and somewhat atypical in appearance, while the nucleus in the stromal cell of giant cell tumor is similar to that of the giant cells: oval with smooth chromatin. Well-formed spicules of lamellar bone are characteristic of clear cell
chondrosarcoma (e-Fig. 4.4), and the nucleus is large and vesicular. Neither giant cell tumor nor clear cell chondrosarcoma exhibit the characteristic “chicken-wire” pattern of matrix calcification seen in chondroblastoma. The features of the epiphyseal tumors are summarized in Table 4.4.
chondrosarcoma (e-Fig. 4.4), and the nucleus is large and vesicular. Neither giant cell tumor nor clear cell chondrosarcoma exhibit the characteristic “chicken-wire” pattern of matrix calcification seen in chondroblastoma. The features of the epiphyseal tumors are summarized in Table 4.4.
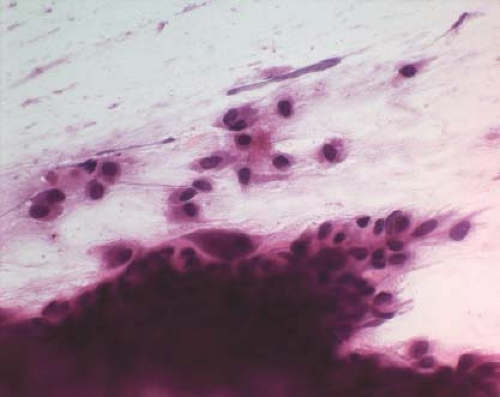 Figure 4.2 Touch preparation revealing hyperchromatic folded nuclei and osteoclast-like giant cells. |
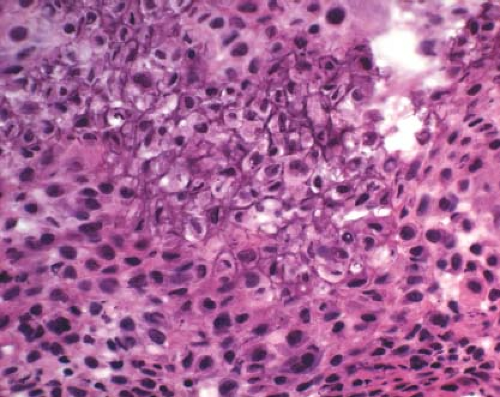 Figure 4.3 Chondroblastoma frequently displays a chicken-wire type of calcification of the fine chondroid matrix produced by the tumor. |
Table 4.4 Features of Epiphyseal Lesions |
|---|