INTRODUCTORY CASE (BULLOUS PEMPHIGOID)
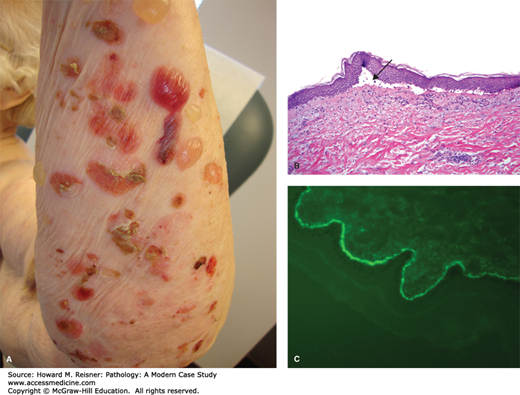
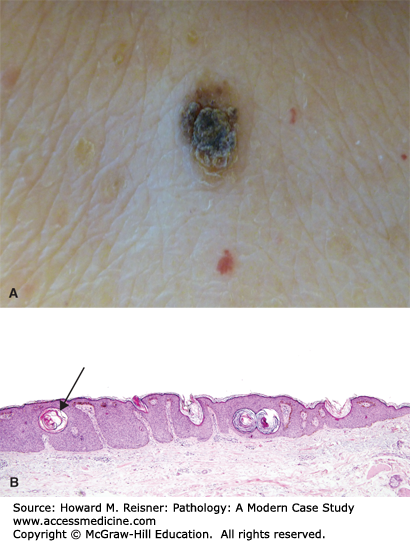
A 77-year-old woman presents to a local emergency room after developing numerous blisters over her trunk and extremities. She reports that the evening prior she developed an intensely pruritic red rash, and the next morning she was alarmed when she woke and noticed large, painful blisters erupting within the rash. Examination shows numerous tense bullae with erythematous, indurated borders over the trunk and extremities. A dermatologist is called for consultation and performs a punch biopsy from the edge of one of the large blisters. Clinical image and results of hematoxylin and eosin (H&E) staining are shown in Figure 20-1A–C.
FIGURE 20-1
Bullous phemphigoid. (A) Tense bullae overlying an erythematous base in bullous pemphigoid. Photograph Courtesy of Dr. Luis Diaz, UNC Dermatology. (B) Hematoxylin and eosin stained biopsy with split at dermal–epidermal junction (arrow). (C) Direct immunofluorescence demonstrating linear staining of IgG at the basement membrane zone. Image used with permission of Dr. Luis Diaz and Dr. Donna Culton, UNC Dermatology.
Microscopic examination is most notable for a split at the dermoepidermal junction. While this finding can be seen in other blistering disorders, it is perhaps most characteristic of bullous pemphigoid. Bullous pemphigoid is an autoimmune blistering disorder caused by formation of pathogenic antibodies to a component of hemidesmosomes, specifically bullous pemphigoid antigen 2, which is necessary for binding of the superficial component of the skin, the epidermis, to the deeper component, the dermis. An accompanying inflammatory infiltrate with prominent eosinophils is also highly indicative of bullous pemphigoid. As discussed in the cases below, further diagnostic studies using immunofluorescence can often help cinch the diagnosis.
PART 1: INTRODUCTION
The skin is the largest organ in the body and serves as its external covering, separating our sometimes fragile interior from the dangers of our environment. It provides a barrier that protects the body from both physical and environmental threats. It also acts as the primary defensive layer of the immune system by preventing infectious organisms from entering the body. Additionally, the skin plays a significant role in thermoregulation and is important in preventing overheating or overcooling of the body.
In medicine, the skin is involved in a vast number of pathological conditions. While there is a large number of conditions that primarily and exclusively manifest in the skin, our external surface can also give us diagnostic clues to pathology taking place elsewhere in the body. Because there are such a large number of conditions affecting the skin, it is sometimes useful to think of the conditions that affect the skin in groups. These may include inflammatory, immune-mediated, neoplastic, and infectious processes. Study of the skin is not the sole realm of the dermatologist as almost every field or subfield in medicine requires at least a basic understanding of dermatopathologic conditions, and it is often the case that certain specialties and subspecialties require an even more in-depth knowledge. For example, an ear, nose, and throat specialist may become an expert regarding basal cell and squamous cell carcinoma, while the gynecologist may develop an important familiarity with lichen planus, lichen sclerosus, and certain types of adnexal tumors affecting the female genital area. Similarly, a gastroenterologist may develop an expertise with a disorder such as dermatitis herpetiformis, which often occurs with the common disorder of gastrointestinal distress, celiac disease.
Here we hope to provide a basic, practical foundation in dermatopathology on which a student can build as they become further specialized. This will serve them in communicating with colleagues from their own specialties and others. Of course, it is not possible for us to discuss each of the protean number of diseases exhaustively. We have thus chosen to highlight diseases for which professionals from most if not all fields require knowledge. Different texts on the subject have divided dermatopathology content in different ways and many disease processes could fit into multiple groups. For simplicity, we have divided the following content into groups based on conditions and prototypes in order to provide an introductory knowledge. We first begin with a general discussion of skin anatomy and histology to serve as a basis for the subsequent sections.
SKIN STRUCTURE
QUICK REVIEW
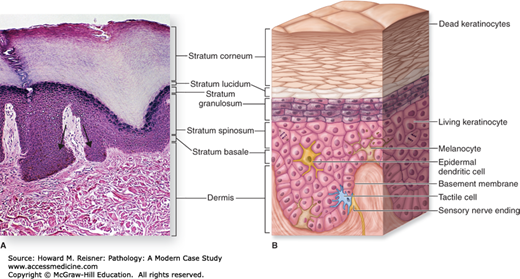
The superficial or external surfaces of the body are generally divided into three zones. These are, from superficial to deep, the epidermis, dermis, and subcutis. The epidermis is the overlying squamous layer and is analogous to epithelial linings that cover other body surfaces, including the mucosal surfaces of the airways, the epithelium overlying the gastrointestinal tract, and the epithelial surfaces in the reproductive and urinary organs. The epidermis is commonly divided into different layers that become useful in describing histological findings. The deepest of these layers is the basal layer (stratum basale) that lines the epidermal side of the dermoepidermal junction, and includes mostly cuboidal-appearing cells called basal cells (aka basal keratinocytes). The basal layer is the site of mitosis or proliferation of skin cells. These cells can sometimes appear slightly more basophilic or blue than more superficial cells, and are connected to the dermis by structures called hemidesmosomes. The basal layer is also home to a second cell type known as melanocytes. Melanocytes are responsible for melanin pigment production and for the transfer of melanin to keratinocytes, where most of the melanin is stored. Melanin pigment helps to protect the skin from the sun (an increase in the amount of melanin in darker skin individuals is the main reason why they burn less readily). Along with the cells of the basal layer, the melanocytes line the deepest layer of the epidermis and occur in a ratio of basal cell to melanocyte of approximately 1:10.
The majority of cells along the basal layer are known as keratinocytes and as they age will mature superficially in the epidermis. They have intercellular connections between cells, known as desmosomes, which become microscopically apparent superficial to the basal layer. It is for this reason that the next layer of the epidermis is known as the spinous layer (stratum spinosum; the desmosomes give a spinous appearance). The desmosomes are responsible for holding keratinocytes together and resisting shearing forces. Just superficial to the spinous layer the cytoplasm of the keratinocytes undergoes slight horizontal elongation and takes on a more granular appearance due to the appearance of purple keratohyalin granules. It is for this reason that cells in this layer are known as granulocytes, which populate the granular layer (stratum granulosum). Exaggeration or attenuation of this layer is an important diagnostic feature in some conditions.
In the most superficial layer of the epidermis, the horizontal elongation of keratinocytes continues and is accompanied by a loss of their nuclei. They also become even less eosinophilic (less pink) and take on a “cleared out” appearance. This layer is called the cornified layer (stratum corneum). This is the most superficial layer and is an important clue not only to the presence of certain types of skin conditions but also to their age. Pathologically, cells of the most superficial layer can take on a more basophilic, less oval appearance, and retain their nuclei. This is known as parakeratosis and is often a clue that not only is disease present, but also that it has been occurring for at least several weeks. Remember that the epidermis develops from superficial to deep and you will understand why evidence of parakeratosis in the superficial layers means that the pathological insult has affected even the “oldest” cells, since cells from the basal layer take 14 days to migrate to the cornified layer, and then an additional 14 days to detach from the epidermis entirely.
Other cell types are also found in the epidermis including antigen presenting or dendritic cells, tactile cells, and cells involved in sensorium.
The whole of the epidermis takes on an undulating texture at low microscopic power and these undulations are known as rete ridges. Changes in the lengths of these rete ridges as well as changes in their appearance can also serve as important diagnostic clues. It is also worth pointing out that not all skin is the same over all body surfaces. For example, skin in “special sites” may have unique appearances and may cause diagnostic difficulties in some cases, especially involving melanocytic lesions. Acral skin or skin on the palms and soles is known to have well-pronounced rete ridges and a thickened cornified layer.
The dermis is the layer of connective tissue and supporting structures that is directly beneath or deep to the epidermis. It is composed of collagen fascicles and elastic fibers that provide the tensile and elastic properties of the skin. The dermis at the level of the rete ridges is known as the papillary dermis, while the dermis deep to the rete is known as the reticular dermis. Changes in the appearance of these collagen fascicles can also provide important clues to the nature of certain diseases. The dermis also is home to the glands of the skin, specifically three types: sebaceous, eccrine, and apocrine. These glands function in sweat and oil production as well as temperature regulation and are closely related to hair follicles in origin and function. They are closely related to hair follicles. Small arrector pili muscles attach to the hair follicles and are responsible for “goose bumps” when they contract and raise the hair. Along with sensory nerves, there are also small superficial and deep vascular layers of the dermis that provide important blood supply to the dermis as well as the epidermis (the epidermis itself is avascular).
Deep to the dermis is the subcutis, which is composed mostly of fat, blood vessels, nerves, and connective tissue (Figure 20-2).
FIGURE 20-2
Skin structure. (A) Hematoxylin and eosin section of normal skin with prominent rete ridge undulations (arrow). (B) Layers and cell types of the epidermis and their relationship to the dermis. (Reproduced with permission from McKinley M, O’Loughlin VD. Human Anatomy, 2nd ed. New York: McGraw-Hill; 2008.)
WHAT TO DO The Curse of History
There are thousands of rashes. Physicians have been looking at skin for centuries, and for most of that time rashes were named and grouped based on clinical appearance. This yielded a burgeoning nomenclature laden with peculiar Greek and Latin names unrelated to pathogenesis. As an example, consider some of the “erythemas:” erythema multiforme, a dermatitis triggered by herpes virus (among other immune triggers) in which distant sites in the epidermis are attacked immunologically; erythema annulare centrifugum, a dermatitis with lesions resembling hives; and erythema nodosum (EN), a panniculitis (inflammation of the subcutaneous fat). The only thing these dermatoses have in common is redness. As with all bad medical nomenclature, the nomenclature of dermatology is firmly entrenched and will remain inviolate.
Why are there so many rashes and skin problems?
You can see your skin, but you cannot see your liver. Therefore, even the most subtle permutations of skin redness and scaliness can be analyzed by both patients and physicians, but inflammation in the liver and most other organ systems remains occult unless organ dysfunction or failure occurs. The skin has an incredibly delicate and complex neural network, with the capability to produce symptoms such as itching and burning. These symptoms motivate the patient and doctor to classify, diagnose, and treat even minor skin diseases. In other (less innervated) organ systems, minor disorders may remain asymptomatic and occult.
Your skin is in constant contact with the environment. Much like the bowel, the skin is exposed to a steady stream of insults from our often-noxious surroundings, which results in countless episodes and variations in immune responses. Other organ systems have a more filtered, and thus safer view of the world.
Biopsy Types and ProcessingA quick word regarding specimen biopsy and processing is in order for the understanding of the student, whether a future clinician or pathologist. There are basically three types of biopsies that are commonly used for removing small lesions or sampling extensive lesions: these are the shave, punch, and excisional biopsies. The type of biopsy chosen often is dependent on the clinical impression. If the clinician suspects that the lesion is a superficial process, a shave biopsy is typically performed. For deeper processes involving the dermis or even subcutis, a punch biopsy is often required. For larger lesions, an excisional biopsy, where the clinician narrowly removes the whole lesion, is the best method of sampling. Since diagnosis of a skin disease often depends on tissue sampling, choice of proper biopsy becomes very important and can sometimes be an art form. Proper and sufficient feedback between clinician and pathologist is important to ensure the tissue is being sampled adequately.
PHYSICAL DESCRIPTIONS FOR PRIMARY SKIN LESIONS
Histological diagnoses of dermatological specimens are rarely made without clinical context and history in mind. Important items for consideration are timing, distribution, and appearance. Primary skin lesions are those that arise as the direct result of a disease process without distortion from outside factors. In order to minimize confusion, clinicians tend to use specific terms. Some important terms are listed in Tables 20-1, 20-2, 20-3.
| Primary lesions demonstrate the natural presentation of a skin lesion that is unadultered by outside forces. |
| Macule – <1 cm flat area of discoloration |
| Patch – >1 cm flat area of discoloration |
| Papule – <1 cm, elevated, palpable skin lesion |
| Plaque – >1 cm, elevated, palpable skin lesion |
| Nodule – A rounded, palpable skin lesion of roughly equal diameter and depth |
| Vesicle – A fluid-filled space (blister), <1 cm in diameter |
| Bulla – A fluid-filled space (blister), >1 cm in diameter |
| Pustule – A pus-filled space |
| Secondary lesions are those that result from primary lesions following influence from extraneous factors or the passage of time. These include the lesions below: |
| Scale – Thin fragments of keratinized stratum corneum |
| Crust – Layer formed from dried serous, purulent, or sanguineous exudate |
| Excoriation – Disruption in the skin as a result of scratching |
| Fissure – Linear clefts through the epidermis +/– the dermis, result of thick, inelastic skin |
| Erosion – Loss of epidermis; heals without scar. Often the remnant of a ruptured bulla |
| Ulcer – Loss of both epidermis and a portion of the dermis, heals with scar |
| Acanthosis – Thickening of the epidermis |
| Acantholysis – Loss of cohesion between keratinocytes of the epidermis |
| Basket weave – Term given to typical appearing keratin layer |
| Dysplasia – Cellular changes that may be indicative of a premalignant or malignant process |
| Parakeratosis – The presence of nuclei in the cornified layer |
| Verrucous – Having a warty architecture |
| Infiltrative – Jagged aggregates of cells invading underlying tissue, often implying a more aggressive phenotype |
| Circumscribed – Well demarcated |
| S100 protein, HMB-45, Melan A – Commonly used to stain melanoma |
| AE1/AE3, cytokeratin, p63 – Commonly used to identify cells of squamous differentiation |
| CD45 – Identifies most hematopoietic cells |
| CD68 – Commonly used to identify macrophages (histiocytes) |
| CD31/34 – Used to identify cells of vascular origin |
| PAS (periodic acid-Schiff)/diastase – Stains a variety of entities including fungi and glucose; diastase sensitivity indicates presence of glucose |
| CD1a – Stains Langerhans cells |
| Ki-67 – Used as a proliferation marker, increased staining with high proliferation rate |
| GMS (Gomori-Grocott methenamine silver stain) – Used to stain fungi |
PART 2: PERTINENT DERMATOLOGICAL CONDITIONS AND CASE PRESENTATIONS
There are several different types of cysts observed in the skin. Features used to classify cysts include the type of epithelium making up the cyst wall, the contents of the cyst, and the types of cells or structures surrounding the cyst. While a detailed discussion of every type of skin cyst is beyond the scope of this text, we do highlight the most common cyst types. The infundibular follicular, commonly known as an epidermal inclusion cyst, is by far the most common type of cyst in the skin. It can occur anywhere, but has a predilection for the head and neck. It is typified histologically by a cyst wall resembling the surface epidermis with a granular layer and a cyst space filled with lamellated keratin. These are also referred to as epidermoid cysts or colloquially as “sebaceous cysts” although they do not have anything to do with the sebaceous glands. Milia are small epidermoid cysts (1–3 mm), which are often seen in newborns on the face, upper trunk, and extremities. They can also be seen after scarring processes.
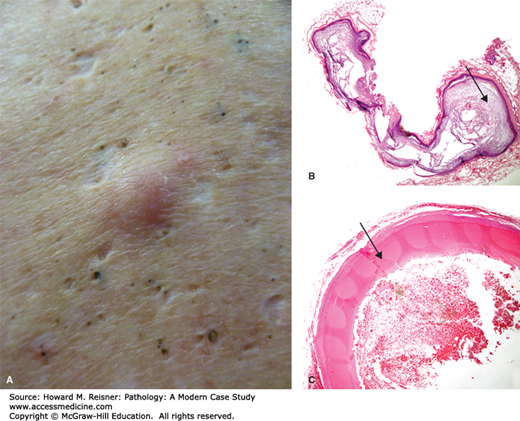
In contrast, the pilar cyst (trichilemmal cyst) is characterized by compact pink keratin surrounded by squamous epithelium lacking a granular layer. It is usually seen as a cyst on the scalp. A related lesion, the pilomatricoma, is benign and occurs in the head and necks of children (Figure 20-3A, B, C).
CASE 20-1
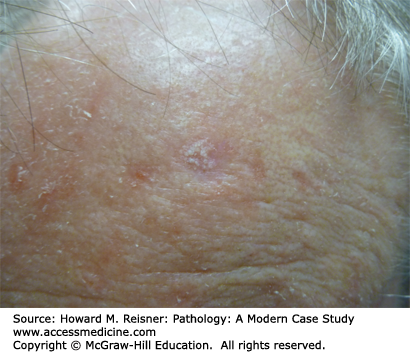
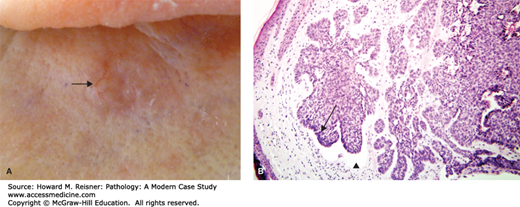
A 72-year-old woman presents with a subtle scaly papule on her forehead, just above the eye. It is painless and is shown in Figure 20-4.
EPIDERMAL LESIONS AND TUMORS
An actinic keratosis is considered a premalignant lesion, typically arising with increasing age and sunlight exposure (Figure 20-4). These are common on sun-exposed areas of older individuals with fair skin types. These lesions are considered to be precancerous (an intermediate to squamous cell carcinoma); however, the overwhelming majority will never progress to cancer. Histologically, these lesions are typified by atypical keratinocytes present within the basal portion of the epidermis. Full-thickness atypia, by definition, warrants the diagnosis of squamous cell carcinoma in situ (Bowen disease).
Seborrheic keratosis is one of the most common tumors of the skin and frequently appears as an incidental finding in older patients. The most common presentation is a waxy, “stuck on” tan-brown papule or small plaque (Figure 20-5), although occasionally they take on a skin-colored or slightly erythematous appearance. They commonly occur on the head, neck, and trunk, with a propensity for areas of friction such as under bra straps or waistbands. Although seborrheic keratoses have no malignant potential, they are often biopsied when confused with other pigmented lesions, or when pruritus or other symptoms arise.
CASE 20-2
A 65-year-old man presents with a 1 cm brown waxy lesion on his forehead. The lesion has a “stuck on” appearance. The specimen is processed and histology is shown in Figure 20-5A, B.
CASE 20-3
A 65-year-old man presents with a pink, shiny papule on his cheek. There is central ulceration of this lesion and telangiectasias. His dermatologist performs a shave biopsy and the light microscopic examination is shown in Figure 20-6A, B.
The appearance of seborrheic keratosis on microscopic examination is somewhat variable, but all cases have in common lack of significant atypia, mitotic figures, cellular pleomorphism, or other characteristics suggestive of more aggressive lesions. Seborrheic keratosis often demonstrates thickening of the epidermis (acanthosis), with a flat, symmetrical base, and horn pseudocysts (called pseudo because they presumably connect to the surface, hence not true cysts).
BASAL CELL CARCINOMA
Basal cell carcinoma is the most common skin tumor in man and occurs most commonly in the areas of the head and neck, but can occur anywhere on the body. Tumors that go untreated for years can directly invade underlying or adjacent structures; however, most that have been treated early do not cause significant problems. Only extremely rare incidences of metastatic disease have been reported. Incidence increases with age and sun exposure, and the vast majority is found in patients with fair skin. Histologically, several classic features typify these lesions. One is a striking basophilia or blue appearance to the tumor cells themselves, and there is often an increase in mitotic rate or necrosis within collections of tumor cells. There is a lining up of the basal cells around the periphery of cellular collections, an appearance commonly referred to as “peripheral palisading.” There is also “clefting” between palisades of tumor cells and the surrounding stroma. This is a separation and contraction of tumor cells from the surrounding stroma during processing and can be an important clue in differentiating this lesion from other similar lesions.
The nevoid basal cell carcinoma syndrome (Gorlin syndrome) is a rare autosomal dominant condition in which patients are predisposed to the development of basal cell carcinomas, especially at an early age, as well as odontogenic keratocysts, abnormal facies, congenital malformations, and systemic malignancy to name a few. This is thought to be due to a mutation in the protein patched homolog 1 tumor suppressor protein (PTCH) located on chromosome 9q.
SQUAMOUS CELL CARCINOMA
The second most common tumor of the skin is squamous cell carcinoma. This tumor can occur in many different locations throughout the body. Cutaneous squamous cell carcinoma occurs more commonly in sun-exposed Caucasian skin and has an increasing incidence with age. This tumor originates from abnormally developing keratinocytes and is typified by atypical keratinocytes, frequently with increased nuclear pleomorphism, abnormal keratinization, frequent mitotic figures, and irregular architecture. This lesion exists in an in situ form, meaning that all of the abnormal tumor cells are limited to the epidermis.
Squamous cell carcinoma with an invasive component, in contrast, is identified by abnormal tumor cells that invade into and through the basement membrane. These tumors are easily confused with other less-threatening entities, especially on limited sampling. Similar to basal cell carcinoma, these lesions can often exist for some time without causing problems for the patient, but local destruction can result. Metastasis is possible in thick lesions. Patients who are immunosuppressed, particularly patients who have a received a transplant, are at particularly high risk of developing many squamous cell carcinomas. Certain lesions predispose to the development of squamous cell carcinoma including actinic keratosis, lichen sclerosus, scars, sites of irradiation, and burns. Patients with epidermodysplasia verruciformis, an autosomal recessive skin disorder resulting in an abnormal susceptibility to human papillomavirus (HPV, see below) infection, are also at higher risk, as well as patients with xeroderma pigmentosum, an autosomal recessive disorder of DNA repair.
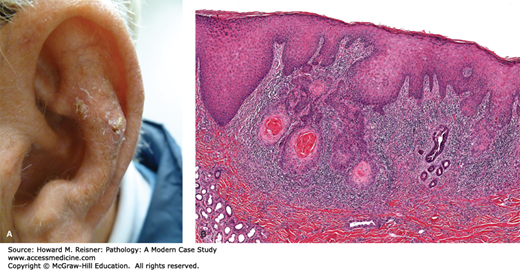
CASE 20-4
A 61-year-old man presents with a 0.5 cm ulcerated and irregular papule on his ear. Histological examination reveals the following Figure 20-7A, B.
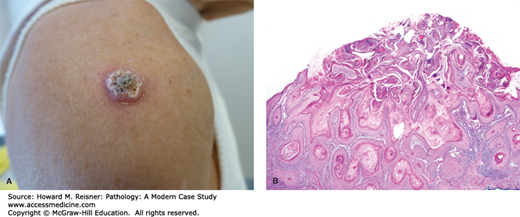
KERATOACANTHOMA
Keratoacanthoma is a neoplasm that commonly occurs on the face of Caucasians. Clinically, this tumor is a keratinized papule that arises and grows quickly over the course of weeks to a few months, and spontaneous regression usually, but not invariably, also occurs over this same timeframe. Given the marked keratinization that is evident both grossly and histologically, some experts think of the tumor as “keratinizing itself to death.” Histologically, this tumor is typified by a central cup-shaped architecture filled with a keratin “plug.” Lesional cells at the base of and surrounding the central keratin “plug” often exhibit abundant eosinophilic (pink) cytoplasm and can demonstrate some cytologic atypia. Because predicting the course of the lesion histologically is difficult, some prefer the term squamous cell carcinoma, keratoacanthoma type (Figure 20-8A, B).
A Note About Solar/Ultraviolet Light Damage
Not only do the tanning (mostly UVA) and burning (mostly UVB) rays of the sun cause actinic keratosis, basal cell carcinoma, and squamous cell carcinoma that manifest when humans are older, they also cause wrinkles that are manifest when humans are relatively young. Even though you and your patients may have already received a significant dose of UV light, it is never too late to begin a healthy relationship with the sun. Tanning salons boast they are safe since they only use UVA spectrum lamps, but these not only cause skin aging but are also linked to increased risk of all skin cancers, including melanoma. Avoid sunburn. Wear sunscreen on a daily basis and use a sunscreen that blocks both UVA and UVB. Wear a hat. Wear sunglasses. While squamous and basal cell carcinomas have traditionally been afflictions of elderly patients, it is becoming more commonly seen in patients in their 20s and 30s with extensive UV exposure history.
PIGMENTED LESIONS
Lesions that arise from the pigment-producing cells of the skin, melanocytes, are known as melanocytic lesions. It is convenient for pathologists to classify lesions involving these cell groups as “pigmented,” although certainly other types of lesions can demonstrate pigment as well. Like lesions arising from any cell type in the body, many benign proliferations of melanocytes exist. A malignant proliferation of melanocytes is called melanoma and is one of the most deadly of all human neoplasms, especially if not detected and treated at an early stage. The most common and significant melanocytic lesions are discussed below.
These are very common lesions that occur most frequently on the face, chest, and shoulders as well as the hands and arms. They occur as multiple red/tan macules scattered over the affected area and are usually 2–3 mm in diameter or less. Histologically, melanocytes in these lesions are generally not increased as it is actually an increased amount of melanin pigmentation in keratinocytes that causes the clinical appearance. Unlike lentigos, which are discussed below, ephelides often fade in the absence of UV exposure and return on future exposure (Figure 20-9).
Similar to freckles, melasma is a common condition that affects the face and is characterized by irregular and confluent hyperpigmentation. Also like freckles, it is characterized histologically by an increase in melanin pigmentation of keratinocytes. It is most frequently seen during pregnancy, is accentuated by UV exposure, and has been associated with oral contraceptives.
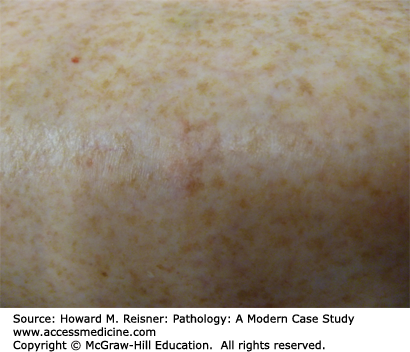
These are common lesions of the elderly, which develop in sun-damaged skin of the arms, hands, and face. They occur clinically as hyperpigmentation in the form of a tan or brown macules or patches. Histologically, they are characterized by elongated and rounded, “club like” rete ridges with prominent excess pigment at the base along with solar elastosis (Figure 20-10).
These are small (less than 5 mm) hyperpigmented macules that can occur anywhere on the body and are often congenital or arise at young age. These lesions are asymptomatic but can be related to a number of syndromes. Histologically, these lesions are characterized by an increase in normal melanocytes along the dermoepidermal junction and an increase in melanin pigment (Figure 20-11).
FIGURE 20-11