Pathology and Treatment of Zenker Diverticulum
Sidhu P. Gangadharan
Zenker diverticulum was initially described by Abraham Ludlow in 1769 when he discovered the pathology during the autopsy of a man who had died from “obstructed deglutition.” This pharyngoesophageal diverticulum was given its eponym after Dr. Friedrich Albert von Zenker who described the pathology in great detail nearly a century later, including the pathophysiologic postulate that it arose as a pulsion diverticulum secondary to high hypopharyngeal pressures during swallowing. Thirty years later, Dr. George Killian, an otolaryngologist, identified the weak area at the transition between hypopharynx and esophagus through which the diverticulum forms, bounded by the inferior pharyngeal constrictor superiorly and cricopharyngeus muscle inferiorly, forming the triangle, which bears his name. It is a false diverticulum, with mucosa prolapsing posteriorly through Killian’s triangle. Typically, as the diverticulum enlarges it will progress inferiorly, giving rise to the endoscopic view of both lumens, with the cricopharyngeus muscle under the mucosa at the bifurcation of the esophagus and diverticulum.
The vast majority of patients who present with a symptomatic Zenker diverticulum will have dysphagia. Regurgitation of undigested food, halitosis, and cervical borborygmi or noisy swallowing is also common. Secondary presenting symptoms include those arising from recurrent or chronic aspiration, such as cough, purulent sputum production, hoarseness, and dyspnea. Compressive effects from a large Zenker diverticulum could include dysphonia or voice changes and neck masses. Cachexia and advanced pulmonary insufficiency suggest significant and long-standing impact from the diverticulum (Table 1).
Typically, the patient is a male in their seventh or eighth decade. This speaks to the acquired nature of this pathology, which is rarely seen in younger individuals. Concomitant gastroesophageal reflux disease is seen at a higher proportion than the normal population, and hiatal hernias may be found in nearly one-quarter. However, it has not been proved that reflux disease itself contributes to the formation of a Zenker diverticulum. Incidental radiographic observation of cervical or thoracic inlet air–fluid levels, tracheal deviation, or mediastinal widening may raise suspicion for a Zenker diverticulum. The incidence of Zenker diverticulum has been reported as high as 1:1,000, though small, asymptomatic, incidentally noted radiographic abnormalities are of unclear clinical significance.
Table 1 Zenker Diverticulum: Clinical Presentation and Symptoms | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
The physical examination of a patient with a Zenker diverticulum varies depending on the chronicity of the disease and magnitude of its physiologic derangement. Thus, halitosis and poor dentition might be observed if regurgitation is a significant presenting complaint. With larger diverticula, Boyce’s sign may be elicited when palpation of the neck results in gurgling as the air- and fluid-filled diverticulum is compressed, similar to the results of palpation of a bowel-filled abdominal wall hernia. Other nonspecific signs of the downstream effects of a symptomatic Zenker diverticulum include pulmonary examination findings of pneumonia or respiratory insufficiency. The differential diagnosis includes other pharyngeal abnormalities such as Killian–Jamieson diverticula (proximal lateral cervical diverticula), esophageal carcinoma, esophageal webs or strictures, foreign body, tracheoesophageal fistula, cricopharyngeal dysfunction without diverticulum, and thyroid pathologies, which might exert local mass effects.
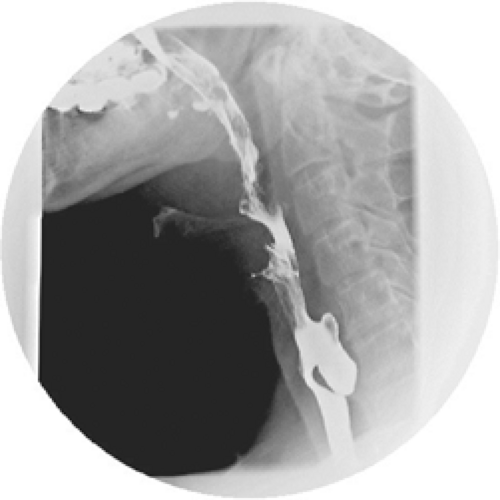
To clinch the diagnosis of a Zenker diverticulum, however, radiographic evidence is most commonly obtained in the form of a barium swallow (Fig. 1). Although dysphagia may more commonly prompt an endoscopic examination, the symptom of regurgitation of undigested food in combination with dysphagia in an elderly patient should lead to a barium swallow as the primary examination. This should demonstrate very adequately the posterior protrusion of the diverticulum, just above the cricopharyngeus muscle. The dimensions of the diverticulum can be determined with a barium swallow, and these dimensions will have implications on the type of treatment that is recommended, as will be discussed later. The possibility of inadvertent perforation of the diverticular lumen during endoscopic examination is another reason given for avoiding this as the primary diagnostic modality for Zenker diverticulum. In addition, these patients are often frail and may present with pulmonary comorbidity, so avoiding an invasive diagnostic procedure may also be desirable.
The pathophysiology of Zenker diverticulum can be observed with manometry. Initial investigation by Ellis in 1969 suggested a discoordination between the pharyngeal and cricopharyngeal contractions—specifically, premature cessation of relaxation of the upper esophageal sphincter, preventing the oral bolus to transit normally into the esophagus. This results in abnormally persistent hypopharyngeal increased pressures against a closed upper esophageal sphincter, which the authors suggested may explain the development of the pulsion diverticulum. Subsequent manometric studies by Cook and colleagues in 1992 have refined this understanding, as they demonstrated increased hypopharyngeal pressures during the transit of the oral bolus and the opening of the upper esophageal sphincter in patients with Zenker diverticulum, but no discoordination of relaxation and contraction of the sphincter. The constrictive physiology of the cricopharyngeus muscle, which resulted in this increased resistance to bolus flow, was substantiated in the publication by that same group of a histologic study of the structural abnormalities of the muscle in patients with Zenker diverticulum. Other researchers have corroborated abnormal histologic findings in the cricopharyngeus muscle of Zenker diverticula patients, and aberrant innervation of the muscle is one proposed theory of the development of these structural abnormalities. Despite these striking findings, it is worth emphasizing that manometry is not utilized in common clinical practice during the workup of Zenker diverticulum.
The development of noninvasive physiologic diagnostic testing for Zenker diverticulum has particular appeal in this elderly patient population. Although barium swallow will delineate the anatomic features noninvasively, it does not clarify the physiologic derangement of the disease. Recently, Vaiman has reported on the utility of surface electromyography in the evaluation of Zenker diverticulum and the results of surgical treatment. Extremely high laryngeal strap muscle activity was noted in the pretreatment of patients with Zenker diverticulum. Following surgery, this activity level decreases to the normal range over the course of several months postoperatively. Other measured parameters that were characteristic for Zenker diverticulum include patterns of regurgitation and duration of swallowing. Although these are preliminary data in small numbers of patients with this pathology, this diagnostic tool may prove useful in diagnosis and follow-up after the treatment of Zenker diverticulum.
Open Surgical Approaches
The most comprehensive operation, and one that many consider the gold standard, is a cricopharyngeal myotomy in combination with a diverticulectomy. This complies with Belsey’s admonition to address the functional aspect of the disease (i.e., the cricopharyngeus muscle) and not simply the result of the abnormal physiology (i.e., the diverticular sac). It should be noted that the data is retrospective and largely single-institution in nature; there exist no randomized prospective trials comparing outcomes of the open surgical outcomes (Table 2).
Cricopharyngeal myotomy with diverticulectomy is most often performed via a left cervical incision, with dissection of the diverticular sac, identification and division of the cricopharyngeus muscle, and then resection of the diverticulum. A drain is usually left in place. The patient is maintained without oral intake until a contrast esophagram is performed. A cautious advancement of diet then proceeds. In our institution, we prefer to transect the diverticular neck above a stapled closure to resect the diverticulum. Both the myotomy and diverticulectomy are performed with the assistance of an esophageal bougie, which helps present the cricopharyngeus muscle and avoids narrowing of the lumen upon closure of the diverticulectomy. We perform the contrast study on the first postoperative day, and we will advance the diet to a soft solid diet over the course of several days, though the patient need not remain hospitalized for that entire 3- or 4-day process. Factors that influence the postoperative variables such as time to oral intake and hospital length of stay include institutional and surgeon preference, debility of the patient, and development of complications.
For very small Zenker diverticula (less than 1 to 2 cm), where there may be no neck conducive to transaction and closure, a myotomy alone may suffice. In addition, some surgeons prefer to routinely perform a myotomy and diverticulopexy—suspending the diverticular sac in an oblique
superior to inferior direction by suturing the apex of the sac to the prevertebral fascia or pharyngeal musculature to allow dependent drainage. The rationale for choosing this technique over diverticulectomy is the avoidance of a suture or staple line on the esophageal mucosa, with its presumptive increased risk of leak.
superior to inferior direction by suturing the apex of the sac to the prevertebral fascia or pharyngeal musculature to allow dependent drainage. The rationale for choosing this technique over diverticulectomy is the avoidance of a suture or staple line on the esophageal mucosa, with its presumptive increased risk of leak.
Table 2 Open Surgical Results | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree