INTRODUCTION
Parvoviruses are the smallest DNA animal viruses. Because of the limited coding capacity of their genome, viral replication is dependent on functions supplied by replicating host cells or by coinfecting helper viruses. Parvovirus B19 is pathogenic for humans and has a tropism for erythroid progenitor cells. It is the cause of erythema infectiosum (“fifth disease”), a common childhood exanthem; of a polyarthralgia-arthritis syndrome in normal adults; of aplastic crisis in patients with hemolytic disorders; of chronic anemia in immunocompromised individuals; and of fetal death. The human bocaviruses have been detected in respiratory specimens from children with acute respiratory disease and in stool samples, but a role in disease is unproven.
PROPERTIES OF PARVOVIRUSES
Important properties of parvoviruses are listed in Table 31-1. It is noteworthy that there are both autonomously replicating and defective parvoviruses.
| Virion: Icosahedral, 18–26 nm in diameter, 32 capsomeres |
| Composition: DNA (20%), protein (80%) |
| Genome: Single-stranded DNA, linear, 5.6 kb, MW 1.5–2.0 million |
| Proteins: One major (VP2) and one minor (VP1) |
| Envelope: None |
| Replication: Nucleus, dependent on functions of dividing host cells |
| Outstanding characteristics: |
| Very simple viruses |
| Human pathogen, B19, has tropism for red blood cell progenitors |
| One genus contains viruses that are replication-defective and require a helper virus |
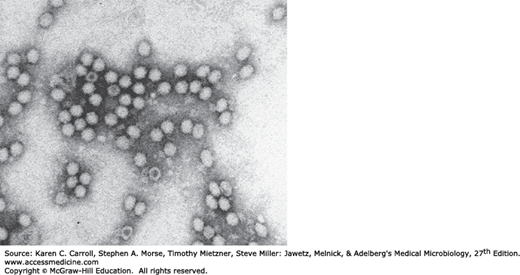
The icosahedral, nonenveloped particles are 18–26 nm in diameter (Figure 31-1). The particles have a molecular weight of 5.5–6.2 × 106 and a heavy buoyant density of 1.39–1.42 g/cm3. Virions are extremely resistant to inactivation. They are stable between a pH of 3 and 9 and withstand heating at 56°C for 60 minutes, but they can be inactivated by formalin, β-propiolactone, and oxidizing agents.
Virions contain two coat proteins that are encoded by an overlapping, in-frame DNA sequence, so that VP2 is identical in sequence to the carboxy portion of VP1. The major capsid protein, VP2, represents about 90% of virion protein. The genome is about 5 kb, linear, single-stranded DNA. Whereas an autonomous virus, B19, contains 5596 nucleotides, a defective parvovirus, AAV-2, contains 4680 bases. Autonomous parvoviruses usually encapsidate primarily DNA strands complementary to viral mRNA; defective viruses tend to encapsidate DNA strands of both polarities with equal frequency into separate virions.
There are two subfamilies of Parvoviridae: the Parvovirinae, which infect vertebrates, and the Densovirinae, which infect insects. The Parvovirinae comprise five genera. Human parvovirus B19 is the most common member of the Erythrovirus genus. There are three human genotypes in this genus. The three human bocaviruses are in the Bocavirus genus. Feline panleukopenia virus and canine parvovirus, both serious causes of veterinary diseases, are classified as members of the Parvovirus genus, as are isolates from many other animals. The genus Dependovirus contains members that are defective and depend on a helper virus (an adenovirus or herpesvirus) for replication. Human “adeno-associated viruses” have not been linked with any disease.
It is difficult to culture human B19 parvovirus. Only primary erythroid progenitors are known to be permissive for B19 infection. The cellular receptor for B19 is blood group P antigen (globoside). P antigen is expressed on mature erythrocytes, erythroid progenitors, megakaryocytes, endothelial cells, placenta, and fetal liver and heart, which helps explain the narrow tissue tropism of B19 virus. The α5β1 integrin is believed to be a coreceptor for B19 entry.
The parvoviruses are highly dependent on cellular functions for replication. Viral DNA replication occurs in the nucleus. The parvoviruses do not have the ability to stimulate resting cells to initiate DNA synthesis, so they must infect dividing cells. One or more cellular DNA polymerases are involved. The nonstructural protein, NS1, is required for virus replication. There are two capsid proteins. Viral replication results in cell death.
PARVOVIRUS INFECTIONS IN HUMANS
A typical course of human parvovirus B19 infection in adults is illustrated in Figure 31-2. B19 has been implicated as the causative agent of several diseases (Table 31-2). Immature cells in the erythroid lineage are principal targets for human B19 parvovirus. Hence, the major sites of virus replication in patients are assumed to be the adult marrow, some blood cells, and the fetal liver. Viral replication causes cell death, interrupting red blood cell production. In immunocompromised patients, persistent B19 infections occur, resulting in chronic anemia. In cases of fetal death, chronic infections may have caused severe anemia in the fetus.
| Syndrome | Host or Condition | Clinical Features |
|---|---|---|
| Erythema infectiosum | Children (fifth disease) Adults | Cutaneous rash Arthralgia-arthritis |
| Transient aplastic crisis | Underlying hemolysis |