INTRODUCTION
The paramyxoviruses include the most important agents of respiratory infections of infants and young children (respiratory syncytial virus [RSV] and the parainfluenza viruses) as well as the causative agents of two of the most common contagious diseases of childhood (mumps and measles). The World Health Organization estimates that acute respiratory infections and pneumonia are responsible every year worldwide for the deaths of 4 million children younger than 5 years. Paramyxoviruses are the major respiratory pathogens in this age group.
All members of the Paramyxoviridae family initiate infection via the respiratory tract. Whereas replication of the respiratory pathogens is limited to the respiratory epithelia, measles and mumps become disseminated throughout the body and produce generalized disease.
Rubella virus, although classified as a togavirus because of its chemical and physical properties (see Chapter 29), can be considered with the paramyxoviruses on an epidemiologic basis.
PROPERTIES OF PARAMYXOVIRUSES
Major properties of paramyxoviruses are listed in Table 40-1.
| Virion: Spherical, pleomorphic, 150 nm or more in diameter (helical nucleocapsid, 13 or 18 nm) |
| Composition: RNA (1%), protein (73%), lipid (20%), carbohydrate (6%) |
| Genome: Single-stranded RNA, linear, nonsegmented, negative sense, ~15 kb |
| Proteins: 6–8 structural proteins |
| Envelope: Contains viral glycoprotein (G, H, or HN) (which sometimes carries hemagglutinin or neuraminidase activity) and fusion (F) glycoprotein; very fragile |
| Replication: Cytoplasm; particles bud from plasma membrane |
Outstanding characteristics: Antigenically stable Particles are labile yet highly infectious |
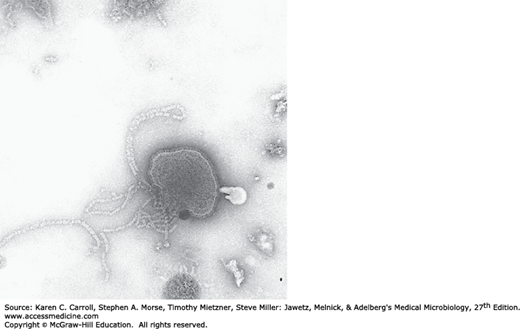
The morphology of Paramyxoviridae is pleomorphic, with particles 150 nm or more in diameter, occasionally ranging up to 700 nm. A typical particle is shown in Figure 40-1. The envelope of paramyxoviruses seems to be fragile, making virus particles labile to storage conditions and prone to distortion in electron micrographs.
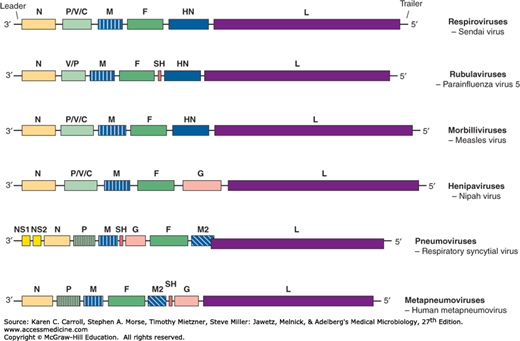
The viral genome is linear, negative-sense, single-stranded, nonsegmented RNA, about 15 kb in size (Figure 40-2). Because the genome is not segmented, this negates any opportunity for frequent genetic reassortment, resulting in antigenic stability.
Most paramyxoviruses contain six structural proteins. Three proteins are complexed with the viral RNA—the nucleocapsid (N) protein that forms the helical nucleocapsid (13 or 18 nm in diameter) and represents the major internal protein and two other large proteins (designated P and L), which are involved in the viral polymerase activity that functions in transcription and RNA replication.
Three proteins participate in the formation of the viral envelope. Matrix (M) protein underlies the viral envelope; it has an affinity for both the N and the viral surface glycoproteins and is important in virion assembly. The nucleocapsid is surrounded by a lipid envelope that is studded with 8- to 12-nm spikes of two different transmembrane glycoproteins. The activities of these surface glycoproteins help differentiate the various genera of the Paramyxoviridae family (Table 40-2). The larger glycoprotein (HN or G) may or may not possess hemagglutination and neuraminidase activities and is responsible for attachment to the host cell. It is assembled as a tetramer in the mature virion. The other glycoprotein (F) mediates membrane fusion and hemolysin activities. The pneumoviruses and metapneumoviruses contain two additional small envelope proteins (M2-1 and SH).
| Paramyxovirinae | Pneumovirinae | |||||
|---|---|---|---|---|---|---|
| Property | Respirovirus | Rubulavirus | Morbillivirus | Henipavirusa | Pneumovirus | Metapneumovirus |
| Human viruses | Parainfluenza 1, 3 | Mumps, parainfluenza 2, 4a, 4b | Measles | Hendra, Nipah | Respiratory syncytial virus | Human metapneumovirus |
| Serotypes | 1 each | 1 each | 1 | Unknown | 2 | Several |
| Diameter of nucleocapsid (nm) | 18 | 18 | 18 | 18 | 13 | 13 |
| Membrane fusion (F protein) | + | + | + | + | + | + |
| Hemolysinb | + | + | + | Unknown | 0 | 0 |
| Hemagglutininc | + | + | + | 0 | 0 | 0 |
| Hemadsorption | + | + | + | 0 | 0 | 0 |
| Neuraminidasec | + | + | 0 | 0 | 0 | 0 |
| Inclusions | C | C | N,C | C | C | C |
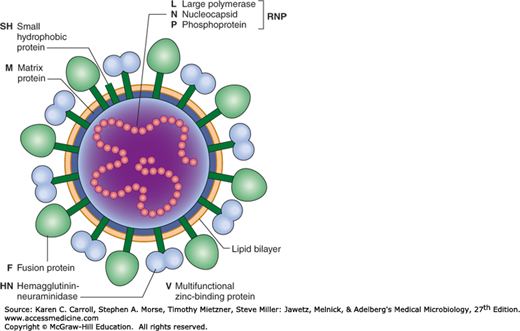
A diagram of a paramyxovirus particle is shown in Figure 40-3.
FIGURE 40-3
Schematic diagram of a paramyxovirus showing major components (not drawn to scale). The viral matrix protein (M) underlies the lipid bilayer. Inserted through the viral membrane are the hemagglutinin–neuraminidase (HN) attachment glycoprotein and the fusion (F) glycoprotein. Only some paramyxoviruses contain the SH protein. Inside the virus is the negative-strand virion RNA, which is encased in the nucleocapsid protein (N). Associated with the nucleocapsid are the L and P proteins, and together this complex has RNA-dependent RNA transcriptase activity. The V protein is only found in rubulavirus virions. (Copyright GD Parks and RA Lamb, 2006.)
The Paramyxoviridae family is divided into two subfamilies and seven genera, six of which contain human pathogens (see Table 40-2). Most of the members are monotypic (ie, they consist of a single serotype); all are antigenically stable.
The genus Respirovirus contains two serotypes of human parainfluenza viruses, and the genus Rubulavirus contains two other parainfluenza viruses as well as mumps virus. Some animal viruses are related to the human strains. Sendai virus of mice, which was the first parainfluenza virus isolated and is now recognized as a common infection in mouse colonies, is a subtype of human type 1 virus. Simian parainfluenza virus 5 (PIV5), a common contaminant of primary monkey cells, is the same as canine parainfluenza virus type 2; shipping fever virus of cattle and sheep is a subtype of type 3. Newcastle disease virus, the prototype avian parainfluenza virus of genus Avulavirus, is also related to the human viruses.
Members within a genus share common antigenic determinants. Although the viruses can be distinguished antigenically using well-defined reagents, hyperimmunization stimulates cross-reactive antibodies that react with all four parainfluenza viruses, mumps virus, and Newcastle disease virus. Such heterotypic antibody responses, which include antibodies directed against both internal and surface proteins of the virus, are commonly observed in older people. This phenomenon makes it difficult to determine by serodiagnosis the most likely infecting type. All members of the genera Respirovirus and Rubulavirus possess hemagglutinating and neuraminidase activities, both carried by the HN glycoprotein, as well as membrane fusion and hemolysin properties, both functions of the F protein.
The Morbillivirus genus contains measles virus (rubeola) of humans as well as canine distemper virus, rinderpest virus of cattle, and aquatic morbilliviruses that infect marine mammals. These viruses are antigenically related to each other but not to members of the other genera. Whereas the F protein is highly conserved among the morbilliviruses, the HN/G proteins display more variability. Measles virus has a hemagglutinin but lacks neuraminidase activity. Measles virus induces formation of intranuclear inclusions, but other paramyxoviruses do not.
The Henipavirus genus contains zoonotic paramyxoviruses that are able to infect and cause disease in humans. Hendra and Nipah viruses, both indigenous to fruit bats, are members of the genus. These viruses lack neuraminidase activity.
Respiratory syncytial viruses of humans and cattle and pneumonia virus of mice constitute the genus Pneumovirus. There are two antigenically distinct strains of RSV of humans, subgroups A and B. The larger surface glycoprotein of pneumoviruses lacks hemagglutinating and neuraminidase activities characteristic of respiroviruses and rubulaviruses, so it is designated the G protein. The F protein of RSV exhibits membrane fusion activity but no hemolysin activity. Human metapneumoviruses are respiratory pathogens of humans classified in the genus Metapneumovirus.
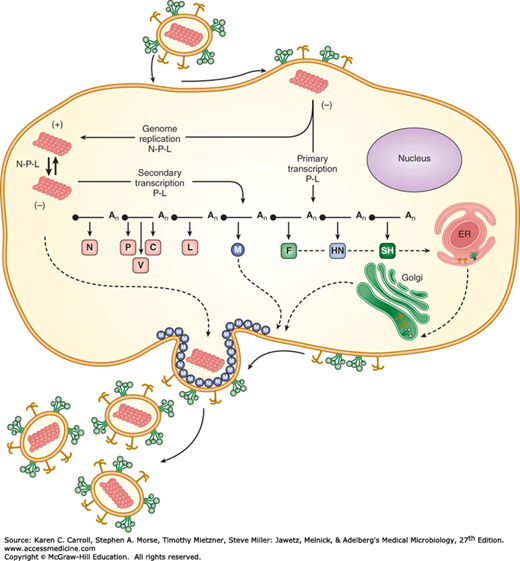
The typical paramyxovirus replication cycle is illustrated in Figure 40-4.
FIGURE 40-4
Paramyxovirus life cycle. The infecting virus particle fuses with the plasma membrane and releases the viral nucleocapsid into the cytoplasm. Solid lines represent transcription and genome replication. Dotted lines indicate transport of newly synthesized viral proteins to plasma membrane. Progeny virions are released from the cell by a budding process. The entire paramyxovirus replication cycle takes place in the cell cytoplasm. ER, endoplasmic reticulum. (Copyright GD Parks and RA Lamb, 2006.)
Paramyxoviruses attach to host cells via the hemagglutinin glycoprotein (HN, H, or G protein). In the case of measles virus, the receptor is the membrane CD46 or the CD150 molecule. Next, the virion envelope fuses with the cell membrane by the action of the fusion glycoprotein F1 cleavage product. The F1 protein undergoes complex refolding during the process of viral and cellular membrane fusion. If the F0 precursor is not cleaved, it has no fusion activity; virion penetration does not occur; and the virus particle is unable to initiate infection. Fusion by F1 occurs at the neutral pH of the extracellular environment, allowing release of the viral nucleocapsid directly into the cell. Thus, paramyxoviruses are able to bypass internalization through endosomes.
Paramyxoviruses contain a nonsegmented, negative-strand RNA genome. Messenger RNA transcripts are made in the cell cytoplasm by the viral RNA polymerase. There is no need for exogenous primers and therefore no dependence on cell nuclear functions. The mRNAs are much smaller than genomic size; each represents a single gene. Transcriptional regulatory sequences at gene boundaries signal transcriptional start and termination. The position of a gene relative to the 3′ end of the genome correlates with transcription efficiency. Whereas the most abundant class of transcripts produced by an infected cell is from the N gene, located nearest the 3′ end of the genome, the least abundant is from the L gene, located at the 5′ end (see Figure 40-2).
Viral proteins are synthesized in the cytoplasm, and the quantity of each gene product corresponds to the level of mRNA transcripts from that gene. Viral glycoproteins are synthesized and glycosylated in the secretory pathway.
The viral polymerase protein complex (P and L proteins) is also responsible for viral genome replication. For successful synthesis of a positive-strand antigenome intermediate template, the polymerase complex must disregard the termination signals interspersed at gene boundaries. Full-length progeny genomes are then copied from the antigenome template.
The nonsegmented genome of paramyxoviruses negates the possibility of gene segment reshuffling (ie, genetic reassortment) so important to the natural history of influenza viruses. The HN/H/G and F surface proteins of paramyxoviruses exhibit minimal antigenic variation over long periods of time. It is surprising that they do not undergo antigenic drift as a result of mutations introduced during replication, because RNA polymerases tend to be error-prone. One possible explanation is that nearly all the amino acids in the primary structures of paramyxovirus glycoproteins may be involved in structural or functional roles, leaving little opportunity for substitutions that would not markedly diminish the viability of the virus.
The virus matures by budding from the cell surface. Progeny nucleocapsids form in the cytoplasm and migrate to the cell surface. They are attracted to sites on the plasma membrane that are studded with viral HN/H/G and F0 glycoprotein spikes. The M protein is essential for particle formation, serving to link the viral envelope to the nucleocapsid. During budding, most host proteins are excluded from the membrane.
The neuraminidase activity of the HN protein of parainfluenza viruses and mumps virus presumably functions to prevent self-aggregation of virus particles. Other paramyxoviruses do not possess neuraminidase activity (see Table 40-2).
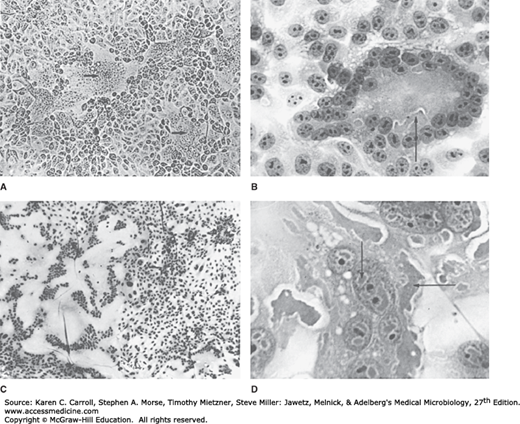
If appropriate host cell proteases are present, F0 proteins in the plasma membrane will be activated by cleavage. Activated fusion protein will then cause fusion of adjacent cell membranes, resulting in formation of large syncytia (Figure 40-5). Syncytium formation is a common response to paramyxovirus infection. Acidophilic cytoplasmic inclusions are regularly formed (see Figure 40-5). Inclusions are believed to reflect sites of viral synthesis and have been found to contain recognizable nucleocapsids and viral proteins. Measles virus also produces intranuclear inclusions (see Figure 40-5).
FIGURE 40-5
Syncytial formation induced by paramyxoviruses. A: Respiratory syncytial virus in MA104 cells (unstained, 100×). Syncytia (arrows) result from fusion of plasma membranes; nuclei are accumulated in the center. B: Respiratory syncytial virus in HEp-2 cells (hematoxylin and eosin [H&E] stain, 400×). Syncytium contains many nuclei and acidophilic cytoplasmic inclusions (arrow). C: Measles virus in human kidney cells (H&E stain, 30×). Huge syncytium contains hundreds of nuclei. D: Measles virus in human kidney cells (H&E stain, 400×). Multinucleated giant cell contains acidophilic nuclear inclusions (vertical arrow) and cytoplasmic inclusions (horizontal arrow). (Used with permission from I Jack.)
PARAINFLUENZA VIRUS INFECTIONS
Parainfluenza viruses are ubiquitous and cause common respiratory illnesses in persons of all ages. They are major pathogens of severe respiratory tract disease in infants and young children. Reinfections with parainfluenza viruses are common.
Parainfluenza virus replication in the immunocompetent host appears to be limited to respiratory epithelia. Viremia, if it occurs at all, is uncommon. The infection may involve only the nose and throat, resulting in a “common cold” syndrome. However, infection may be more extensive and, especially with types 1 and 2, may involve the larynx and upper trachea, resulting in croup (laryngotracheobronchitis). Croup is characterized by respiratory obstruction caused by swelling of the larynx and related structures. The infection may spread deeper to the lower trachea and bronchi, culminating in pneumonia or bronchiolitis, especially with type 3, but at a much lower frequency than that observed with RSV.
The duration of parainfluenza virus shedding is about 1 week after onset of illness; some children may excrete virus several days prior to symptoms. Type 3 may be excreted for up to 4 weeks after onset of primary illness. This persistent shedding from young children facilitates spread of infection. Prolonged viral shedding may occur in children with compromised immune function and in adults with chronic lung disease.
Factors that determine the severity of parainfluenza virus disease are unclear but include both viral and host properties, such as susceptibility of the protein to cleavage by different proteases, production of an appropriate protease by host cells, immune status of the patient, and airway hyperreactivity.
The production of virus-specific IgE antibodies during primary infections has been associated with disease severity. The mechanism may involve release of mediators of inflammation that alter airway function.
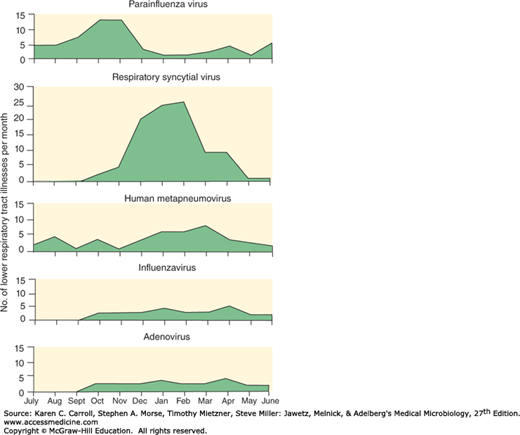
The relative importance of parainfluenza viruses as a cause of respiratory diseases in different age groups is indicated in Table 30-5. Their presence in lower respiratory tract infections in young children shows seasonal variation seen in Figure 40-6.
FIGURE 40-6
Patterns of lower respiratory tract infections in infants and young children with paramyxoviruses and other viruses. Data from 25 years of surveillance (1976–2001) involving 2009 children from birth to age 5 years. (Reproduced with permission from Williams JV, Harris PA, Tollefson SJ, et al: Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004; 350:443–450. Copyright © 2004 Massachusetts Medical Society.)
Primary infections in young children usually result in rhinitis and pharyngitis, often with fever and some bronchitis. However, children with primary infections caused by parainfluenza virus type 1, 2, or 3 may have serious illness, ranging from laryngotracheitis and croup (particularly with types 1 and 2) to bronchiolitis and pneumonia (particularly with type 3). The severe illness associated with type 3 occurs mainly in infants younger than the age of 6 months; croup or laryngotracheobronchitis is more likely to occur in older children between ages 6 months and 18 months. More than half of initial infections with parainfluenza virus type 1, 2, or 3 result in febrile illness. It is estimated that only 2–3% develop into croup. Parainfluenza virus type 4 does not usually cause serious disease, even on first infection.
The most common complication of parainfluenza virus infection is otitis media.
Immunocompromised children and adults are susceptible to severe infections. Mortality rates after parainfluenza infection in bone marrow transplant recipients range from 10% to 20%.
Newcastle disease virus is an avian paramyxovirus that produces pneumoencephalitis in young chickens and respiratory disease in older birds. In humans, it may produce inflammation of the conjunctiva. Recovery is complete in 10–14 days. Infection in humans is an occupational disease limited to workers handling infected birds.
Parainfluenza virus types 1, 2, and 3 are distinct serotypes that lack significant cross-neutralization (see Table 40-2). Virtually all infants have maternal antibodies to the viruses in serum, yet these antibodies do not prevent infection or disease. Reinfection of older children and adults also occurs in the presence of antibodies elicited by an earlier infection. However, those antibodies modify the course of disease; such reinfections usually present simply as nonfebrile upper respiratory infections (colds).
Natural infection stimulates appearance of immunoglobulin A (IgA) antibody in nasal secretions and concomitant resistance to reinfection. The secretory IgA antibodies are most important for providing protection against reinfection but disappear within a few months. Reinfections are thus common even in adults.
Serum antibodies are made to both HN and F viral surface proteins, but their relative roles in determining resistance are unknown. As successive reinfections occur, the antibody response becomes less specific because of shared antigenic determinants among parainfluenza viruses and mumps virus. This makes it difficult to diagnose the specific paramyxovirus associated with a given infection using serologic assays.
Nucleic acid amplification tests are the preferred diagnostic methods because of their sensitivity and specificity, their ability to detect a broad range of viruses, and the rapidity of results.
Antigen detection methods are also useful for rapid diagnosis. The immune response to the initial parainfluenza virus infection in life is type specific. However, with repeated infections, the response becomes less specific, and cross-reactions extend even to mumps virus. Definitive diagnosis relies on viral isolation from appropriate specimens.
Reverse transcription polymerase chain reaction (RT-PCR) assays can be used to detect viral RNA in nasopharyngeal swabs, washes or aspirates, or lower respiratory tract specimens such as bronchoalveolar lavage fluid. Sequence analyses are useful in molecular epidemiology studies of parainfluenza virus infections.
Detection of viral antigens can be done in exfoliated nasopharyngeal cells by direct or indirect immunofluorescence tests. These methods are fairly rapid and simple to perform but are limited by low sensitivity and the range of viruses detected.
Rapid cell culture methods can detect a number of respiratory viruses able to be cultured in vitro but are slower to provide results than nucleic acid or antigen detection methods and are not able to easily detect mixed infections. A continuous monkey kidney cell line, LLC-MK2, is suitable for isolation of parainfluenza viruses. Prompt inoculation of samples into cell cultures is important for successful viral isolation because viral infectivity drops rapidly. For rapid diagnosis, samples are inoculated onto cells growing on coverslips in shell vials and are incubated. One to 3 days later, the cells are fixed and tested by immunofluorescence. Another way to detect the presence of virus is to perform hemadsorption using guinea pig erythrocytes. Depending on the amount of virus, 10 days or more of incubation may be necessary before the cultures become hemadsorption positive. Virus culture is necessary if a viral isolate is desired for research purposes.
Serodiagnosis should be based on paired sera. Antibody responses can be measured using neutralization, hemagglutination-inhibition (HI), or enzyme-linked immunosorbent assay (ELISA) tests. A fourfold rise in titer is indicative of infection with a parainfluenza virus, as is the appearance of specific IgM antibody. However, because of the problem of shared antigens, it is impossible to be confident of the specific virus type involved.
Parainfluenza viruses are a major cause of lower respiratory tract disease in young children (see Figure 40-6). Parainfluenza viruses are widely distributed geographically. Type 3 is most prevalent, with about two-thirds of infants infected during the first year of life; virtually all have antibodies to type 3 by age 2 years. Infections with types 1 and 2 occur at a lower rate, reaching prevalences of about 75% and 60%, respectively, by 5 years of age.
Type 3 is endemic, with some increase during the spring; types 1 and 2 tend to cause epidemics during the fall or winter, frequently on a 2-year cycle.
Reinfections are common throughout childhood and in adults and result in mild upper respiratory tract illnesses. Reportedly, 67% of children are reinfected with parainfluenza type 3 during the second year of life. Reinfections may necessitate hospitalization of adults with chronic lung diseases (eg, asthma).
Parainfluenza viruses are transmitted by direct person-to-person contact or by large-droplet aerosols. Type 1 has been recovered from air samples collected in the vicinity of infected patients. Infections can occur through both the nose and the eyes.
Parainfluenza viruses are usually introduced into a group by preschool children and then spread readily from person to person. The incubation period appears to be from 5 to 6 days. Type 3 virus especially will generally infect all susceptible individuals in a semiclosed population, such as a family or a nursery, within a short time. Parainfluenza viruses are troublesome causes of nosocomial infection in pediatric wards in hospitals. Other high-risk situations include day care centers and schools.
Contact isolation precautions are necessary to manage nosocomial outbreaks of parainfluenza virus. These include restriction of visitors, isolation of infected patients, and gowning and handwashing by medical personnel.
The antiviral drug ribavirin has been used with some benefit in treatment of immunocompromised patients with lower respiratory tract disease.
No vaccine is available.
RESPIRATORY SYNCYTIAL VIRUS INFECTIONS
RSV is the most important cause of lower respiratory tract illness in infants and young children, usually outranking all other microbial pathogens as the cause of bronchiolitis and pneumonia in infants younger than 1 year. It is estimated to account for approximately 25% of pediatric hospitalizations caused by respiratory disease in the United States.
RSV replication occurs initially in epithelial cells of the nasopharynx. Virus may spread into the lower respiratory tract and cause bronchiolitis and pneumonia. Viral antigens can be detected in the upper respiratory tract and in shed epithelial cells. Viremia occurs rarely if at all.
The incubation period between exposure and onset of illness is 3–5 days. Viral shedding may persist for 1–3 weeks from infants and young children, but adults shed virus for only 1–2 days. High viral titers are present in respiratory tract secretions from young children. Inoculum size is an important determinant of successful infection in adults (and possibly in children as well).
An intact immune system seems to be important in resolving an infection because patients with impaired cell-mediated immunity may become persistently infected with RSV and shed virus for months.
Although the airways of very young infants are narrow and more readily obstructed by inflammation and edema, only a subset of young babies develops severe RSV disease. It has been reported that susceptibility to bronchiolitis is genetically linked to polymorphisms in innate immunity genes.