Papilloma, Large Duct and Small Duct
Key Facts
Terminology
Large duct papilloma (LDP)
Usually centrally located; often solitary
Originates in lactiferous sinus or large mammary ducts
Small duct papilloma (SDP)
Usually peripherally located; smaller lesions involving terminal ductal lobular units
Often multiple (papillomatosis)
Epithelium more likely to show foci of ADH or DCIS compared with LDP
Clinical Issues
LDP may present with pathologic nipple discharge
Larger lesions may be palpable
Standard treatment for LDP is complete excision
Benign lesions on excision need no further surgical treatment
Solitary LDPs have an increased relative risk of developing breast carcinoma (1.5-2.0x)
Risk is slightly higher for women with multiple peripheral SDP (papillomatosis)
Microscopic Pathology
Arborizing fronds of tissue with well-developed central fibrovascular core
Lined by epithelial cells, myoepithelial cell layer
Top Differential Diagnoses
Papillary DCIS
Encapsulated (intracystic) carcinoma
Solid papillary carcinoma
Nipple adenoma
TERMINOLOGY
Abbreviations
Large duct papilloma (LDP)
Small duct papilloma (SDP)
Synonyms
Central papilloma
Peripheral papilloma
Intraductal papilloma
Definitions
Benign epithelial proliferative lesions characterized by papillary ingrowths into major ducts (LDP) or smaller ducts (SDP)
CLINICAL ISSUES
Epidemiology
Age
LDP: Most frequent in women 35-50 years old
SDP: Usually younger
Site
LDP: Centrally located in subareolar lactiferous ducts; usually solitary
SDP: Peripherally located; often multiple (“papillomatosis”)
Presentation
LDP
Nipple discharge present in 80% of cases
In women < 60, 7% of cases with discharge are associated with malignancy
In women > 60, 30% of cases with discharge are associated with malignancy
Nipple discharge associated with pathologic lesions is unilateral and spontaneous
Sanguinous or serosanguinous: 70%
Bloody (less common): May be due to papilloma twisting on stalk and infarction
Other causes: Duct ectasia, mastitis, cysts, carcinoma (especially papillary and micropapillary DCIS)
Palpable subareolar mass
May form a lobulated mass on mammography
SDP
Finding on screening mammography
Incidental finding in a biopsy for another lesion
Usually does not cause discharge or a palpable mass
Treatment
Surgical approaches
Symptomatic papillomas are excised for diagnosis and treatment of nipple discharge
For benign lesions on excision, no further surgical treatment is necessary
Prognosis
Papillomas are benign
Mild increased risk of subsequent carcinoma: 1.5-2.0x relative risk or ˜ 5-7% lifetime risk
Risk similar to that for moderate or florid ductal epithelial hyperplasia
Classified as proliferative disease without atypia
Breast cancer risk is slightly higher for women with multiple peripheral SDP (papillomatosis)
Can occur in either breast and at any site
Core Needle Biopsies
Usually fragmented and can be difficult to evaluate
IHC can be helpful in difficult cases
Confirm the presence of myoepithelial cells at periphery and in fibrovascular cores
Cytokeratin 5/6 is generally positive in benign papillomas and absent in papillary carcinomas
Excision may be warranted in the following situations
Large (˜ 2 cm) &/or palpable lesions
Papillomas with nuclear atypia
Papillomas partially involved by proliferations that would be diagnostic of ADH or DCIS if outside of the papilloma
Management of lesions diagnosed as benign papillomas on core needle biopsy is controversial
Risk of carcinoma on excision of benign papillomas is very low
When cases are carefully selected and there is good radiologic/pathologic correlation, carcinomas on excision are absent or rare (< 5%)
However, distinction between benign papillomas and atypical papillomas can be difficult, and some authorities recommend excision of all papillary lesions on core needle biopsy
Papillomas with atypia should be excised as 20-60% of cases will reveal carcinoma on excision
IMAGE FINDINGS
Mammographic Findings
LDP may not be visible by mammography
SDP can present as lobulated mass or cluster of calcifications
Ultrasonographic Findings
LDP: Intraductal, well-defined, hypoechoic mass near nipple
May have both solid and cystic components
Adjacent ducts often dilated
SDP: Small circumscribed or lobulated masses
Ductography
LDP associated with nipple discharge may be diagnosed by ductography
Involved ductal orifice is often dilated
Very difficult to cannulate a duct in the absence of discharge
Contrast agent can show 1 or multiple filling defects with smooth contours in the duct
Papilloma interrupts flow of contrast
Ductography may help localize lesion for excision
MACROSCOPIC FEATURES
General Features
Excisions for nipple discharge require special processing
May lack a mass detectable by palpation or imaging
Surgeon excises subareolar tissue beneath duct orifice with discharge
Dilated duct should be marked with a suture
Involved duct is opened longitudinally and examined for gross lesions
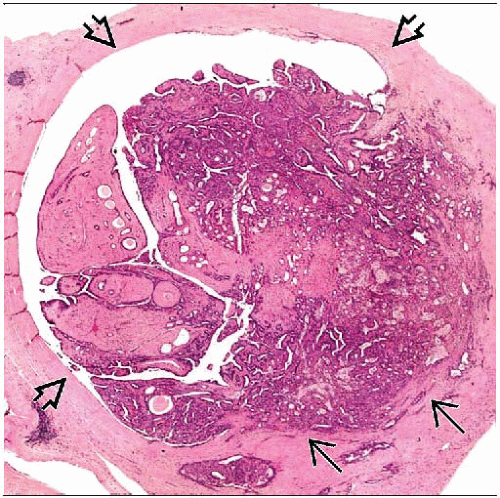
LDP may be visible macroscopically
Appears as tan-pink, circumscribed nodule protruding into a dilated duct or cystically dilated space
Cystic spaces may be filled with serosanguinous fluid and hemorrhage
Verrucous, bosselated, or frankly papillary appearance
Many papillomas, including the majority of SDPs, are not evident on gross exam
Excisions for palpable masses or mammographically detected lesions do not require special processing
Size
LDP is usually < 2 cm but can be as large as 4-5 cm
SDP is usually < 1 cm
MICROSCOPIC PATHOLOGY
Histologic Features
LDP
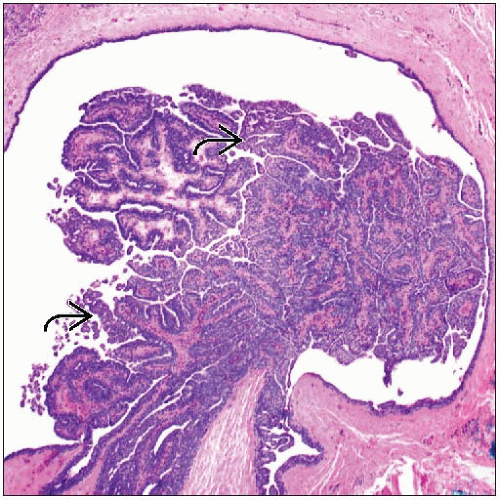
Originates in lactiferous sinus or large mammary ducts
Arborizing finger-like fronds of fibrovascular stromal digitations
Covered by luminal epithelial cells with associated myoepithelial cells
Presence of myoepithelial cells and their distribution in lesion is helpful diagnostic feature
May require use of myoepithelial markers to aid in the diagnostic evaluation in problematic cases
Apocrine metaplasia may be present and is supportive of benign diagnosis
Epithelial hyperplasia can be present and may be florid
“Sclerosing papilloma” refers to LDP with extensive fibrosis of fibrovascular cores &/or lesions with associated sclerosing adenosis
Squamous metaplasia may be present
Rarely, spindle or squamous cell carcinomas can arise in association with papillomas
Infarction, necrosis, and hemorrhage can occur if the LDP twists on its stalk
SDP
Usually peripherally located small lesions involving terminal ductal lobular units
Often multiple (papillomatosis)
Fibrovascular cores are usually well defined
May show varying degrees of fibrosis and sclerosis
Demonstration of myoepithelium by IHC may be helpful for problematic cases
Fibrosis may entrap benign glands and solid epithelial nests
Entrapped glands may mimic infiltrating carcinoma
Papilloma with atypia
2 types
Entire papilloma appears atypical
Focal areas within papilloma fulfill criteria for ADH or DCIS
Atypical features in an entire papilloma include
Monomorphic-appearing epithelial cells
Complex architectural patterns (e.g., cribriform, micropapillary, or solid)
Thin, delicate fibrovascular cores
Absence of apocrine metaplasia or squamous metaplasia
IHC to demonstrate a myoepithelial cell layer is helpful to exclude papillary carcinoma
Surrounding tissue should be examined to find areas of carcinoma away from the papilloma
Papillomas with focal atypical areas are also seen
Criteria for diagnosing DCIS within a papilloma vary
It has been suggested that DCIS should not be diagnosed if area is < 0.3 cm or < 30% of papilloma
However, others favor diagnosing DCIS if criteria for diagnosis would be fulfilled if outside of the papilloma
Presence of DCIS in surrounding breast tissue is helpful to support diagnosis of DCIS and is more significant for determining patient’s risk of subsequent breast cancer
Clinical significance of DCIS limited to a papilloma and completely excised is unclear; risk of subsequent breast cancer is at least equivalent to a diagnosis of ADH; multiple lesions may also increase risk
IHC for high molecular weight cytokeratins (CK5/6) can be helpful to distinguish hyperplasia (patchy positivity) from ADH (negative) or DCIS (negative)
Spindle cell carcinoma arising in papilloma
Squamous metaplasia may occur in papillomas, especially if associated with trauma or infarction
Spindle cell carcinomas can arise adjacent to these areas
Spindle cells may be difficult to distinguish from reactive fibroblasts in fibrous capsule of papilloma
Nuclear atypia and mitoses may be present
IHC for keratin (particularly basal types) and p63 is helpful to establish epithelial origin of spindle cells
Infarction
Extensive coagulative necrosis can be seen in papillomas
May be focal or involve entire lesion
Can be associated with prior needle biopsies or twisting of stalk
Can result in a clinical bloody discharge
Completely necrotic papillary lesions can be difficult to classify
Epithelial displacement
Entrapment of benign epithelium in core needle biopsy sites, surgical sites, or other areas of stromal disturbance occurs most frequently with papillary lesions
Benign cells can be pushed into lymphatics and be seen in sentinel node
Usually, a few cells are present and would be classified as isolated tumor cells
IHC can be helpful in many cases to demonstrate presence of both myoepithelial and epithelial cells
If myoepithelial cells are absent, diagnosis of invasive carcinoma &/or metastatic carcinoma should be made with great caution if artifactual displacement is a possibility
ANCILLARY TESTS
Immunohistochemistry
Myoepithelial markers
Benign papillomas usually have prominent myoepithelial cell layer
IHC for myoepithelial cells can be helpful to document their presence
Papillary carcinomas lack myoepithelial cells
p63 is most helpful for evaluation of fibrovascular cores
Both myoepithelial cells and endothelial cells are positive for muscle markers
Blood vessels can be closely opposed to basal portion of epithelial cells and can be misidentified as myoepithelial cells
Cytokeratin 5/6
Florid hyperplasia in papillomas can be difficult to distinguish from ADH or DCIS
Hyperplasia usually shows patchy positivity for cytokeratin 5/6, whereas ADH and DCIS are generally negative
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree