High-Yield Terms to Learn
 -Glucosidase An enzyme in the gastrointestinal tract that converts complex starches and oligosaccharides to monosaccharides; inhibited by acarbose and miglitol Beta (B) cells in the islets of Langerhans Insulin-producing cells in the endocrine pancreas Hypoglycemia Dangerously lowered serum glucose concentration; a toxic effect of high insulin concentrations and the secretagogue class of oral antidiabetic drugs Lactic acidosis Acidemia due to excess serum lactic acid; can result from excess production or decreased metabolism of lactic acid Type 1 diabetes mellitus A form of chronic hyperglycemia caused by immunologic destruction of pancreatic beta cells Type 2 diabetes mellitus A form of chronic hyperglycemia initially caused by resistance to insulin; often progresses to insulin deficiency
-Glucosidase An enzyme in the gastrointestinal tract that converts complex starches and oligosaccharides to monosaccharides; inhibited by acarbose and miglitol Beta (B) cells in the islets of Langerhans Insulin-producing cells in the endocrine pancreas Hypoglycemia Dangerously lowered serum glucose concentration; a toxic effect of high insulin concentrations and the secretagogue class of oral antidiabetic drugs Lactic acidosis Acidemia due to excess serum lactic acid; can result from excess production or decreased metabolism of lactic acid Type 1 diabetes mellitus A form of chronic hyperglycemia caused by immunologic destruction of pancreatic beta cells Type 2 diabetes mellitus A form of chronic hyperglycemia initially caused by resistance to insulin; often progresses to insulin deficiency
Diabetes Mellitus
Two major forms of diabetes mellitus have been identified. Type 1 diabetes usually has its onset during childhood and results from autoimmune destruction of pancreatic B cells. Type 2 diabetes is a progressive disorder characterized by increasing insulin resistance and diminishing insulin secretory capacity. Type 2 diabetes is frequently associated with obesity and is much more common than type 1 diabetes. Although type 2 diabetes usually has its onset in adulthood, the incidence in children and adolescents is rising dramatically, in parallel with the increase in obesity in children and adolescents.
The clinical history and course of these 2 forms differ considerably, but treatment in both cases requires careful attention to diet, fasting and postprandial blood glucose concentrations, and serum concentrations of hemoglobin A1c, a glycosylated hemoglobin that serves as a marker of glycemia. Type 1 diabetes requires treatment with insulin. The early stages of type 2 diabetes usually can be controlled with noninsulin antidiabetic drugs. However, patients in the later stages of type 2 diabetes often require the addition of insulin to their drug regimen.
Insulin
Physiology
Insulin is synthesized as the prohormone proinsulin, an 86-amino-acid single-chain polypeptide. Cleavage of proinsulin and cross-linking result in the 2-chain 51-peptide insulin molecule and a 31-amino-acid residual C-peptide. Neither proinsulin nor C-peptide appears to have any physiologic actions.
Effects
Insulin has important effects on almost every tissue of the body. When activated by the hormone, the insulin receptor, a transmembrane tyrosine kinase, phosphorylates itself and a variety of intracellular proteins when activated by the hormone. The major target organs for insulin action include:
Liver
Insulin increases the storage of glucose as glycogen in the liver. This involves the insertion of additional GLUT2 glucose transport molecules in cell plasma membranes; increased synthesis of the enzymes pyruvate kinase, phosphofructokinase, and glucokinase; and suppression of several other enzymes. Insulin also decreases protein catabolism.
Skeletal Muscle
Insulin stimulates glycogen synthesis and protein synthesis. Glucose transport into muscle cells is facilitated by insertion of GLUT4 transporters into cell plasma membranes.
Adipose Tissue
Insulin facilitates triglyceride storage by activating plasma lipoprotein lipase, increasing glucose transport into cells via GLUT4 transporters, and reducing intracellular lipolysis.
Insulin Preparations
Human insulin is manufactured by bacterial recombinant DNA technology. The available forms provide 4 rates of onset and durations of effect that range from rapid-acting to long-acting (Figure 41-1). The goals of insulin therapy are to control both basal and postprandial (after a meal) glucose levels while minimizing the risk of hypoglycemia. Insulin formulations with different rates of onset and effect are often combined to achieve these goals.
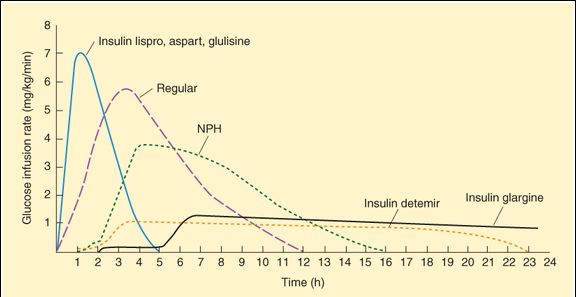
FIGURE 41-1
Extent and duration of action of various types of insulin as indicated by the glucose infusion rates (mg/kg/min) required to maintain a constant glucose concentration. The durations of action shown are typical of an average dose of 0.2-0.3 U/kg; the duration of regular and NPH insulin increases considerably when dosage is increased.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 41-5.)
Rapid-Acting
Three insulin analogs (insulin lispro, insulin aspart, and insulin glulisine) have rapid onsets and early peaks of activity (Figure 41-1) that permit control of postprandial glucose levels. The 3 rapid-acting insulins have small alterations in their primary amino acid sequences that speed their entry into the circulation without affecting their interaction with the insulin receptor. The rapid-acting insulins are injected immediately before a meal and are the preferred insulin for continuous subcutaneous infusion devices. They also can be used for emergency treatment of uncomplicated diabetic ketoacidosis.
Short-Acting
Regular insulin is used intravenously in emergencies or administered subcutaneously in ordinary maintenance regimens, alone or mixed with intermediate- or long-acting preparations. Before the development of rapid-acting insulins, it was the primary form of insulin used for controlling postprandial glucose concentrations, but it requires administration 1 h or more before a meal.
Intermediate-Acting
Neutral protamine Hagedorn insulin (NPH insulin) is a combination of regular insulin and protamine (a highly basic protein also used to reverse the action of unfractionated heparin, Chapter 34) that exhibits a delayed onset and peak of action (Figure 41-1). NPH insulin is often combined with regular and rapid-acting insulins.
Long-Acting
Insulin glargine and insulin detemir are modified forms of human insulin that provide a peakless basal insulin level lasting more than 20 h, which helps control basal glucose levels without producing hypoglycemia.
Insulin Delivery Systems
The standard mode of insulin therapy is subcutaneous injection with conventional disposable needles and syringes. More convenient means of administration are also available.
Portable pen-sized injectors are used to facilitate subcutaneous injection. Some contain replaceable cartridges, whereas others are disposable.
Continuous subcutaneous insulin infusion devices avoid the need for multiple daily injections and provide flexibility in the scheduling of patients’ daily activities. Programmable pumps deliver a constant 24-h basal rate, and manual adjustments in the rate of delivery can be made to accommodate changes in insulin requirements (eg, before meals or exercise).
Hazards of Insulin Use
The most common complication is hypoglycemia, resulting from excessive insulin effect. To prevent the brain damage that may result from hypoglycemia, prompt administration of glucose (sugar or candy by mouth, glucose by vein) or of glucagon (by intramuscular injection) is essential. Patients with advanced renal disease, the elderly, and children younger than 7 years are most susceptible to the detrimental effects of hypoglycemia.
The most common form of insulin-induced immunologic complication is the formation of antibodies to insulin or noninsulin protein contaminants, which results in resistance to the action of the drug or allergic reactions. With the current use of highly purified human insulins, immunologic complications are uncommon.
Noninsulin Antidiabetic Drugs
Four well-established groups of oral antidiabetic drugs are used most commonly to treat type 2 diabetes. These include insulin secretagogues, the biguanide metformin, thiazolidinediones, and  -glucosidase inhibitors (Figure 41-2). Three novel agents—pramlintide, exenatide, and sitagliptin—target endogenous regulators of glucose homeostasis. The durations of action of important members of these groups are listed in Table 41-1.
-glucosidase inhibitors (Figure 41-2). Three novel agents—pramlintide, exenatide, and sitagliptin—target endogenous regulators of glucose homeostasis. The durations of action of important members of these groups are listed in Table 41-1.
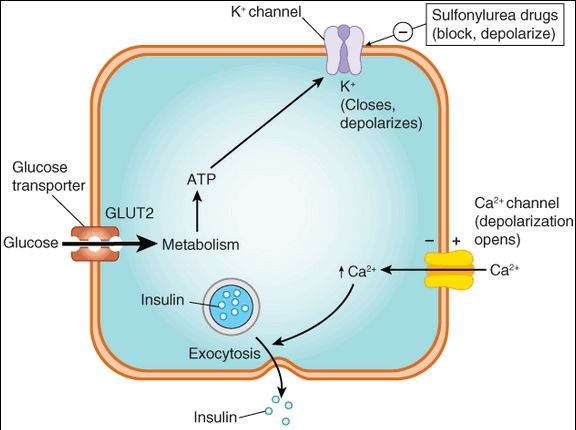
FIGURE 41-2
Control of insulin release from the pancreatic beta cell by glucose and by sulfonylurea drugs. When the extracellular glucose concentration increases, more glucose enters the cell via the GLUT2 glucose transporter and leads, through metabolism, to increased intracellular ATP production with subsequent closure of ATP-dependent K+ channels, membrane depolarization, opening of voltage-gated Ca2+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree