Packaging
Sudaxshina Murdan
Chapter contents
Key points
• The closure, such as a stopper, lid, top or cap, is an integral part of the pack.
• A pack can be single-unit, e.g. a sachet, or multiple-unit, e.g. a bottle containing many tablets.
Introduction
Packaging is the means of economically providing containment, protection, presentation, identification, information, convenience and compliance, for a product during storage, distribution, display and use.
Pharmaceutical packaging enables the packaged medicine’s requirements of efficacy, safety, uniformity, reproducibility, integrity, purity and stability to be maintained throughout the product’s shelf-life. Furthermore, some pharmaceutical packaging is essential for the product’s use and the drug’s administration, i.e. the pack is the delivery device. Examples of this are pressurized metered dose inhalers, nasal sprays, transdermal patches and pre-filled syringes.
The role of packaging seems to be constantly expanding, for example, in anti-counterfeiting, branding and providing distinguishing features to the drug product to avoid errors as well as in the monitoring of patient adherence.
The pharmaceutical pack
A pharmaceutical pack contains, protects and delivers a safe, efficacious drug product. At the same time it provides identification and information, enabling patient compliance and convenience. The primary pack, which is in direct contact with the product during storage and delivery (e.g. a glass bottle and cap), contains the product, while the secondary pack (e.g. a carton box for a glass bottle) contains the primary pack, as well as ancillary components, such as dispensing spoons and information leaflets.
Primary packs
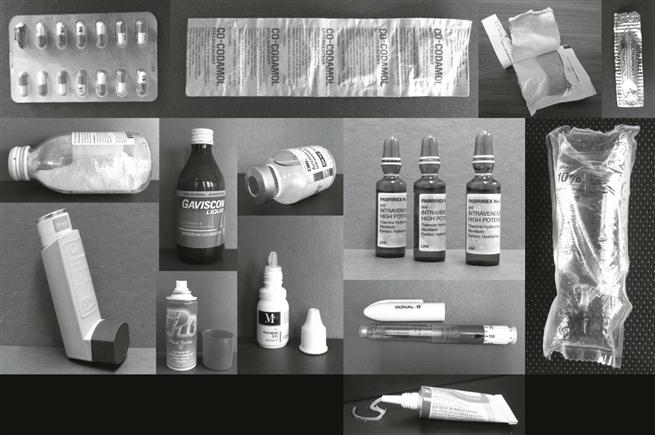
The wide range of pharmaceutical products, such as solid powders, granules, tablets, capsules, semi-solids (e.g. creams, ointments, gels), liquids (such as solutions, suspensions, emulsions), some of which are sterile, obviously require a great diversity, both in primary pack design and in packaging materials. The latter include paper, glass, plastics, rubber, metal or combination materials such as laminates. Examples of primary packs include blister packs, strip packs, sachets, bottles, ampoules, vials, bags, tubes and syringes. The primary pack may contain many doses (i.e. be a multiple-unit pack, e.g. a bottle containing many tablets) or a single dose (i.e. be a single-unit pack, e.g. blister pack, sachet). Examples of the range of pharmaceutical primary packs are shown in Figure 47.1.
The primary pack must offer child-resistance to restrict children’s access to the product. Child-resistant packs have been successful in reducing accidental poisoning of children. At the same time, the pack must allow access to the user, who may be elderly or frail and may have difficulty opening packs. Difficult-to-open packs are not always fully reclosed between administration events – and this may compromise the product. In addition, the primary pack must be tamper-resistant and tamper-evident to improve the product’s security from pilferage and deliberate contamination and safeguard the product’s legitimate user. Ideally, packs would be completely tamper-proof, though this is probably impossible to achieve against determined malice.
The primary pack must be compatible with the product, and take nothing out of the product and add nothing in. Drug absorption or adsorption into the pack would reduce product potency, while chemicals leaching out of the pack and into the product could induce drug degradation. In addition, the primary pack must protect the product against atmospheric factors, such as extremes of temperature, light, moisture, oxygen, carbon dioxide, particulates (e.g. dust, dirt), as well as biological hazards, such as microorganisms, insects and rodents, and enable product stability.
Drug molecules can undergo chemical reactions triggered by light, heat, moisture, or atmospheric gases, such as oxygen (see Chapters 48 for more details). For example, light can provide the energy necessary for a drug isomer to change its configuration. Protection from light is usually achieved by using an opaque or amber-coloured container. Oxygen can cause drug degradation via oxidation. Carbon dioxide can dissolve in the water in unbuffered aqueous products, and lower their pH by forming carbonic acid. Water can cause drug degradation via hydrolysis. Moisture gain into a product can also cause dilution of liquid products, wetting of solid products and an aqueous environment can support microbial growth. Solvent loss from a product can also occur if the container is permeable. Secondary packs also contribute to protection against atmospheric factors to some extent, although their major role is to provide protection against mechanical hazards, such as shock (e.g. when dropped), compression, vibration, abrasion, puncture, etc., during handling, storage and transport.
Closures
A closure is a device – e.g. stopper, lid, top or cap – which is used to close a container, and is an integral part of the pack. The word ‘pack’ therefore covers both the container and the closure. Without the latter, the functions of a pack, such as containment, presentation, protection and convenience, cannot be fulfilled, and like the container, the closure must be inert, compatible with the contents, and protect the latter against environmental hazards, such as oxygen, light, moisture, etc.
Certain closures must maintain sterility, e.g. in multi-use parenteral vials. A good seal between the container and the closure prevents anything from leaking out or gaining access into the pack, and is obtained by a snug fit between the inner face of the closure and the external face of the container finish. Resilient liners inside the closure are sometimes used to achieve a snug fit, although many plastic closures are internally moulded to achieve a good seal and are liner-free.
The closure has to be user-friendly, allow easy opening to legitimate consumers and be easy to reclose (for multi-unit packs), as well as child-resistant, tamper-resistant and tamper-evident. Closures may also include dispensing devices, e.g. a pump on bottles containing creams. The outer surface of the closure may also be ribbed to allow good grip when opening by twisting. Pharmaceutical closures are mostly made of plastic (thermosets and thermoplastics), although metal is also used, e.g. on parenteral vials.
The word ‘closure’ does not always refer to a stopper-type device. Metal tubes have two closures – a cap at one end while the other end of the tube is sealed by folding and crimping. Flexible packaging, such as pouches, sachets and blister packs do not contain a closure as defined above. They are instead sealed by heat and/or pressure, or with adhesives, and are non-reclosable packs.
Packaging materials
Once the function(s) of the desired packaging has been defined, selection of the primary packaging material is the first step in the packaging process, and takes into account the dosage form, the route of administration, drug/product stability, need for terminal sterilization and for visual inspection of the packaged medicine, patient compliance and convenience, aesthetics, cost, environmental-friendliness, etc. Liquids, which are in constant intimate contact with the primary pack, as opposed to solids such as tablets and capsules, require greater quality from a pack in order to ‘take nothing out of the product or add nothing in’.
Injectable liquids require even greater quality from the pack compared to oral liquids, to maintain sterility and freedom from other possible contaminants, such as extraneous particulates. Medicines which are terminally sterilized in their final packs need to be made from materials that can withstand the sterilization procedure.
Semi-solids need to be able to be dispensed from the container, under slight pressure, e.g. squeezing of a tube. Drugs which are sensitive to atmospheric conditions such as sunlight need a packaging material which stops light transmission into the pack. Patient convenience and compliance can be aided by the use of blister packaging, which allows transport of the required doses (rather than the whole bottle) and which can also be printed with information to aid memory, for example, for contraceptive pills.
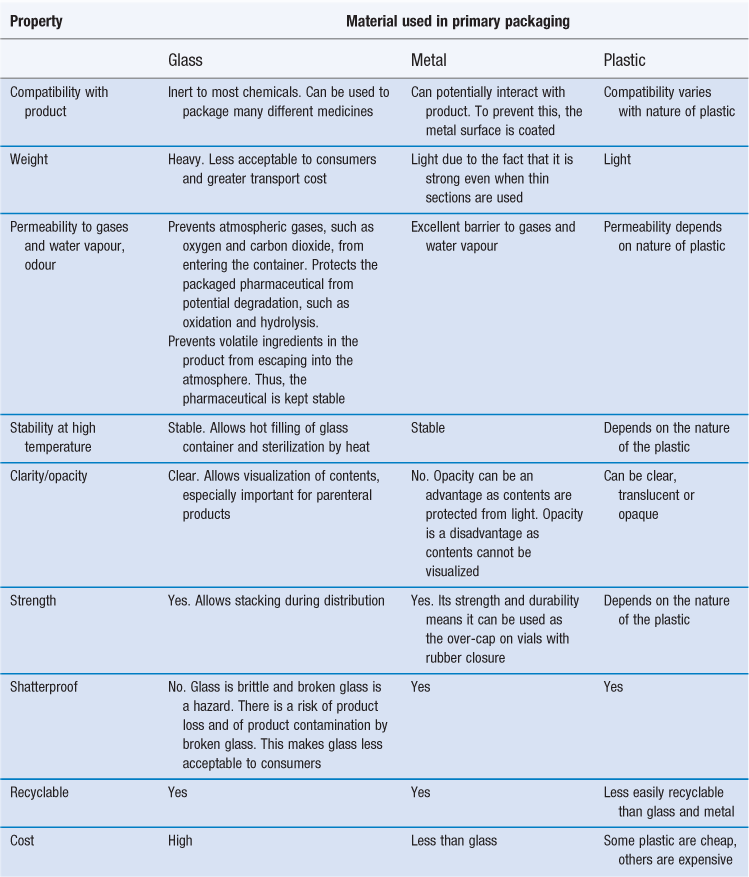
The packaging material is obviously very important and the different materials are described next. Glass, metal and plastic are the materials most commonly used in primary packs; their properties with respect to their use in packaging are compared in Table 47.1.
Glass
Glass – believed to have been first discovered around 3000 BC in the East Mediterranean and which has been used for thousands of years – is widely used for packaging pharmaceuticals due to its excellent barrier properties, inertness and compatibility with pharmaceuticals. Its many advantages are shown in Table 47.1, and it has traditionally been the gold standard in pharmaceutical packaging.
Glass is produced by heating together various inorganic substances to form a molten mass, and then rapidly cooling the latter which solidifies in a non-crystalline state. Sand (or more properly silicon dioxide; silica) which is the main constituent of sand), limestone (calcium carbonate) and soda ash (sodium carbonate) are heated to very high temperatures (e.g. 1500 °C) in a furnace. The ingredients melt and gradually react and form a homogeneous molten mass. The latter is then converted into glass containers by one of two basic methods: blow moulding or tubular glass fabrication. Blow moulding, the older method is when a ‘gob’ (a small piece of highly viscous molten glass) of glass is moulded into a container. The second method is used to manufacture ampoules and vials. Once a homogeneous molten glass mass is formed at high temperature, the molten mass is converted into glass tubing as it moves out of the furnace. The tubing is cut to proscribed lengths and the individual glass tubes are then converted into ampoules or vials.
In addition to silica, soda ash and limestone, other compounds are added in trace amounts to achieve certain properties in the glass formed. For example:
• alumina (Al2O3) increases the hardness, durability and clarity of the glass
• selenium or cobalt oxides improve clarity
• lead oxide gives clarity and sparkle (but makes glass soft)
• boron compounds give low thermal expansion and high heat-shock resistance
• arsenic trioxide and sodium sulphate are added to reduce blisters in the glass.
Opacity and different colours are achieved by the inclusion of a range of compounds, as shown in Table 47.2. The different colours convey specific properties, for example:
• amber glass is widely used to package pharmaceuticals susceptible to degradation by sunlight
• green glass is mostly used for packaging beverages
• blue glass makes white products appear whiter
• opaque white (opal) conveys prestige to upmarket toiletries and cosmetics.
Table 47.2
Additives that are generally used to achieve different colours of glass
| Colour | Possible additives |
| Amber | Iron oxides, manganese oxides, carbon oxides, sulphur compounds |
| Browns |